See the original article online at: https://dev2.apsf.org/article/perioperative-brain-health-its-not-all-positive-attitude-exercise-and-superfoods/
Introduction
Thirty-five years after the creation of the Anesthesia Patient Safety Foundation (APSF), we recall Macintosh’s adage that no patient should be harmed by anesthesia.1 Articulated over 60 years ago, this concept set the cornerstone of the APSF, which codified our calling to safety, vigilance, and the endless pursuit of safe outcomes. At that time, the goal was clear—to address measurable events such as cardiac arrest, hypoxia, and human error. While the above are critically important, the future of patient safety is much more expansive. Let’s begin with a definition of patient safety that we have modified from Gaba and Weinger:*
Safety is how we deliver care in a way that prevents harm from the processes of care, and the behavior of the humans embedded in the system of care. Safety is an emergent property of the system that occurs when we actively try to achieve it.*
*David Gaba and Matthew Weinger presented at the APSF Board Meeting; permission granted for adaptation and citation by personal communication.
David Gaba, MD, and Jeffrey Cooper, PhD, articulate that the foundation of our past success emerged from our trust in standards and guidelines, technological solutions, human factors, and the institutionalism of safety.2,3 We assert that our specialty is at the frontier of patient care, addressing what matters most to our patients: their “healthspan.” We work as teams throughout the episode of perioperative care and beyond to return patients home with improved functional, psychological, and cognitive health.
The pursuit to combat postoperative delirium (POD)—a most surreptitious villain—is elusive and less defined, yet a formidable foe of our specialty’s safety initiatives. Admittedly, we have gaps to fill regarding a comprehensive understanding of the pathophysiology of POD, diagnosis and identification, and tools to advance monitoring and treatment. We require resources for research and an implementation strategy to improve neurocognitive outcomes after surgery.
As perioperative physicians, we cannot ignore the magnitude of POD. The aging demographic of the United States population predicts that more than one-third of our patients will be older than 65. In these patients, POD has an estimated incidence ranging from 5–50% contributing to the $150 billion of delirium-associated health care expenditures in the United States.4 Finally, many of these cases are thought to be preventable through care pathways and best practice.4
Standards & Guidelines and Technology
As Gaba and Cooper note, the history of anesthesiology’s success in attaining a six-sigma safety level in ASA1 patients is in large part attributable to our specialty’s adherence to guidelines and standard operating procedures.3 Two recent consensus statements guide our current understanding of POD. The 2018 Perioperative Brain Health Initiative Summit Report5 identified our current understanding for predisposing risk factors including baseline cognitive decline or dementia, poor vision, poor hearing, severe illness and underlying infection. Although the pathophysiology of POD is not well defined and no definite biomarker currently exists, interrelated mechanisms including neurotransmitter imbalance, inflammation, stress response, cellular metabolism, pre-existing neurologic vulnerability and changes in network neurobiology (Figure 1) may explain why the surgical episode of care contributes to its incidence and severity of results.6
Both the American Society of Anesthesiologists Perioperative Brain Health Initiative and the 2015 American Geriatrics Society Guidelines7 recommend cognitive screening as a pre-surgical measure and metric of risk prior to and after surgery. Preoperatively, many experts advocate for use of the Mental Status Exam or a shortened version of this assessment tool (MMSE or mini-cognition questionnaire seen in Figure 2). A variety of tools for POD diagnosis are available, each with tradeoffs of receiver operator characteristics, including the Confusion Assessment Method (CAM), the CAM-ICU, Nursing Delirium Screening Scale, or Delirium Symptom Interview.8 Yet, abbreviated training often results in imprecise diagnostic rates for a condition that is known to wax and wane in severity within one surgical admission. While convergence on use of a single tool does not exist, both groups recommend additional training and experience in POD diagnostic tools for frontline staff.
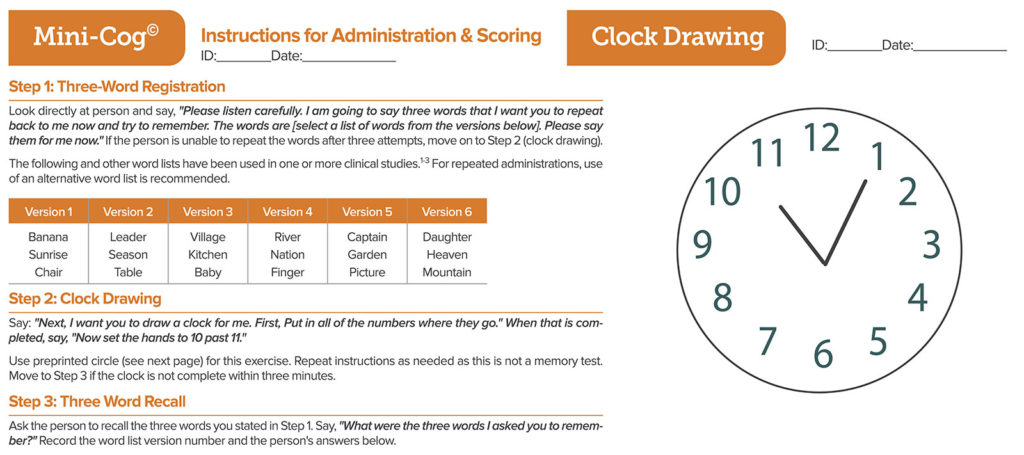
Figure 2: The Mini-Cog test. There are two Mini-Cog® components that include a score for accuracy of “clock drawing” and “three-word recall,” resulting in a cumulative score that can increase the detection of cognitive impairment. There are a total of five possible points for the test with three possible points for the three-word recall and two points for a normal clock. A total score of three or greater indicates a lower likelihood of cognitive impairment. Mini-Cog® copyright, Dr. Soo Borson (used with permission). See mini-cog.com for more detail.
Current strategies for prevention of POD include minimal use of high-risk drugs including benzodiazepines, anticholinergic medications, higher dose corticosteroids, meperidine, and polypharmacy in general. Current literature advocates for non-pharmacologic treatment measures as a first step but urges restraint for antipsychotic medications unless the patient poses potential for self-harm or harm to others.
Anesthesiology has achieved many safety goals using engineering and human factors in the design of instrumentation and monitoring. With this history in mind, we have continued to explore technological solutions towards reducing POD. Our specialty has developed specialized monitoring for cerebral blood flow and EEG-based monitoring to try to reduce the depth of general anesthetics. While early data suggested that excessive anesthetic depth may predispose to POD,9 findings from the recent ENGAGES trial10 do not support this hypothesis and weigh against recent guidelines.7
The Gaps in Our Research—the Role of the APSF
The brain is the target end-organ for general anesthesia. Neurocognitive recovery after surgery is not always a straightforward process, nor is it well understood. Nonetheless, the demand for surgical services will continue, and our engagement in best perioperative practices for neurocognitive health is critical. As such, we should take a leadership role for optimizing brain health for surgical patients.
Fortunately, our field is scientifically and clinically well positioned to address brain health knowledge gaps. We have the ability to track neuroinflammatory signatures for delirium in human participants using basic science models.11 Network neuroscience approaches allow study of brain-state transitions relating to levels—and contents—of consciousness. When translated to clinical settings, preliminary analyses have identified neurophysiologic signatures associated with delirium.12 Thus, opportunities to advance neuroscience related to pathologic brain states across the clinical spectrum, which may also contribute to the fundamental understanding of cognitive dysfunction, extend value beyond the perioperative setting. Lastly, as perioperative neuroscience matures, the time is ripe to probe implementation barriers for interventions that aim to optimize perioperative brain health.13
What Do We Do Today? A Role for Implementation Science and Quality Improvement
Christian Guay, MD, and Michael Avidan, MD, recently argued brain health and POD should not be considered a single syndrome nor treated as such.14 Rather, it is likely a collection of disparate disorders that share some common features. The most compelling, reproducible interventions target multiple modifiable risk factors. These interventions, similar to the Hospital Elder Life Program, mitigate cognitive and functional decline in older hospitalized patients using cognitive orientation, social support, sleep protocols, mobilization, and education for health care staff (Table 1). Until scientific research compels more precise interventions, we need to apply traditional methods of quality improvement, implementation science, and quality control from engineering science and weave modifiable risk factor prevention into our clinical workflows.
Table 1: Proposed Interventions To Mitigate Cognitive & Functional Decline
| Intervention | Description |
| Core Intervention | |
| Daily visitor/orientation | Orientation board with names of care team members and schedule |
| Therapeutic activities | Cognitive stimulation three times daily |
| Early mobilization | Ambulation or active range-of-motion exercises three times daily |
| Vision protocol | Visual aids and adaptive equipment |
| Hearing protocol | Portable amplifying devices and special communication techniques |
| Oral volume repletion | Feeding and drinking assistance and encouragement |
| Sleep enhancement | Nonpharmacologic sleep protocols |
| Program Interventions | |
| Geriatric nursing assessment | Nursing assessment and intervention for cognitive and functional impairment |
| Interdisciplinary rounds | Twice-weekly rounds to discuss patients and set goals |
| Provider education | Formal didactic sessions, one-on-one interactions |
| Community linkages | Referrals and communication with community agencies to optimize transition to home |
| Geriatrician consultation | Targeted consultation referred by program staff |
| Interdisciplinary consultation | As needed consultation upon referral by staff |
Adapted with permission from Inouye SK, Bogardus Jr ST, Baker, DI, et al. The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48:1697-1706.
First, frontline perioperative clinicians should commit to measuring cognitive function prior to surgery. Simple cognitive tools such as the Mini-Cog test (Figure 2) can be applied across primary care, anesthesiology, and geriatric clinics prior to elective surgery. These tools not only provide process data to establish a baseline measurement for the individual patient, but may also serve as population data for longitudinal studies. In her discussion at the Perioperative Brain Health lecture for the APSF in 2018, Deborah Culley, MD, showed the audience how quickly the Mini-Cog can be deployed without clinic workflow changes.
Second, while the precision of existing assessment tools for PODs are lacking, we should instill delirium assessment into the regular practice of frontline clinicians especially for geriatric patients and others at increased POD risk. Recurring, scheduled education should be the norm to maintain clinician familiarity with these tools and prevent protocol adherence drift. By codifying the act of search and diagnosis, we can eventually replace first generation tools with more robust clinical assessment tools.
Third, perioperatively, we can affect human factors changes, such as medication simplification, identification of vision and hearing deficits in the early postoperative course, and minimization of sedation. None of these proposed changes involve substantial capital expenditures nor complex practice redesign, and these interventions can be bundled into our daily work routines to achieve patient-centered goals for the elderly.
Finally, rather than focusing upon highly specific outcome measures required of research science, POD interventions should employ implementation science measurements. We may benefit by utilizing performance improvement tools such as control charts and process measurements to measure diagnostic, monitoring, and therapeutic change, rather than relying on outcome measures until a reliable and valid diagnostic biomarker for POD or more specific therapeutics are developed.
Conclusion
Thirty-five years ago, the APSF articulated its mission that “no patient should be harmed by anesthesia.” Over time, major advances toward prevention of cardiovascular collapse, hypoxemia, drug error, and human error emerged from the organization that made industry-changing improvements to anesthesia safety. These efforts are now engaged at a new frontier of perioperative brain health in order to prevent POD and return patients to their baseline cognitive function or better. In an era of a neuroscience revolution, the APSF has the high-stakes task to address the public health problem of POD. The costs are high; the science around pathophysiology, prevention and treatment has large gaps to traverse; and the workflows need standardization. We look forward to our specialty supporting the discovery of new knowledge that will be the foundation for the implementation science to codify our actions and conquer this next frontier.
Nirav Kamdar is an assistant clinical professor in the Department of Anesthesiology and Perioperative Medicine at UCLA Health, Los Angeles, CA.
Phillip Vlisides is an assistant professor in the Department of Anesthesia and the Center for Consciousness Science at the University of Michigan Medical School, Anne Arbor, MI.
Dan Cole is a professor of Clinical Anesthesiology in the Department of Anesthesiology and Perioperative Medicine at UCLA Health, Los Angeles, CA.
The authors have no conflicts of interest.
References
- Macintosh RR. Deaths under anesthetics. Br J Anaesth. 1949; 21:107–36.
- Gaba DM. Anaesthesiology as a model for patient safety in health care. BMJ. 2000;320:785–8.
- Cooper JB, Gaba D. No myth: anesthesia is a model for addressing patient safety. Anesthesiology. 2002;97:1335–7.
- Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168:27–32
- Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br J Anaesth. 2019;123:464–78.
- Vlisides P, Avidan M. Recent advances in preventing and managing postoperative delirium. F1000Res. 2019;8.
- Postoperative delirium in older adults: best practice statement from the American Geriatrics Society – ScienceDirect at https://www.sciencedirect.com/science/article/pii/S1072751514017931?via%3Dihub Last Accessed August 7, 2020.
- Greer N, Rossom R, Anderson P. Delirium: Screening, Prevention, and Diagnosis—A Systematic Review of the Evidence. VA-ESP Project #09-009 2011:95.
- Chan MTV, Cheng BCP, Lee TMC, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. 2013;25:33–42.
- Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery. JAMA. 2019;321:473–83.
- Vasunilashorn SM, Ngo LH, Chan NY, et al. Development of a dynamic multi-protein signature of postoperative delirium. J Gerontol A Biol Sci Med Sci. 2019;74:261–268.
- Tanabe S, Mohanty R, Lindroth H, et al. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66.
- Balas MC, Burke WJ, Gannon D, et al. Implementing the ABCDE Bundle into everyday care: opportunities, challenges and lessons learned for implementing the ICU Pain, Agitation and Delirium (PAD) guidelines. Crit Care Med. 2013;41:S116–27.
- Guay CS, Avidan MS. No brain Is an island. Anesth Analg. 2020;130:1568–1571.


 Issue PDF
Issue PDF