In 2006 the ASPF first addressed the issue of drug-induced respiratory depression in the postoperative period,1 and in June of 2011 convened its second conference to work toward mitigating and eventually eliminating this serious patient safety risk.2 During the conference it was evident that there is a significant need for the anesthesia community to better understand and fully embrace the critical importance of continuous respiratory monitoring—particularly capnography in conjunction with, or as an alternative to, pulse oximetry when parenteral opioids are used in the postoperative period. Conference participants generally recommended pulse oximetry for all patients receiving PCA therapy, and capnography only for those receiving supplemental oxygen.3
Until recently capnography monitoring could only be used with intubated patients in the operating suite and intensive care unit (ICU). Most general-care clinicians are not as familiar with this type of monitoring as they are with pulse oximetry. In particular, the value of using capnography to measure not only respiratory rate (RR) but also end-tidal carbon dioxide (EtCO2) is not well recognized.
Overdyk et al. used both pulse oximetry and non-invasive capnography to continuously monitor 178 patients receiving PCA therapy. The incidence of respiratory depression as measured by oxygen desaturation was 12%, consistent with previous estimates.4 However, the incidence of respiratory depression as measured by bradypnea was far greater than the 1 to 2% reported in the literature.5-7 The use of continuous capnography monitoring yielded the following incidences: respiratory depression based on bradypnea, defined traditionally (≥1, two-minute or longer low-RR event [RR < 10 bpm]) was 58%; defined conservatively (≥1, three-minute or longer low-RR event) was 41%.4
Detection of a patient’s declining respiratory status before progression to respiratory depression can help avert undesirable outcomes and transfer to an ICU.8 Because patients vary greatly in their response to opioids, patient status can change quickly, and traditional approaches to respiratory monitoring are less than optimal.
Current monitoring protocols typically require nurses to document the RR and less commonly the oxygen saturation (SpO2) value initially every 30 minutes, then as infrequently as every 2 to 4 hours.9 The nurse’s presence may stimulate the patient, resulting in overestimation of the resting RR, which is often determined by manual respiration counts. Manual counts have been shown to be inaccurate when compared to capnometry.10 Nurses usually are not available for continuous monitoring, and there is no automated alarm to alert nurses not in the room.
Even at a low RR, oxygen saturation is usually maintained.11 Lethal hypercarbia is possible despite normal oxygen saturation.2 As a result, pulse oximetry may fail to detect respiratory deterioration, particularly if a patient is receiving supplemental oxygen, which delays the progression of respiratory failure from bradypnea to apnea.11 Thus, even continuous monitoring of heart rate and SpO2 by pulse oximetry is not a substitute for monitoring RR, EtCO2, and apneic events by capnography.12
A growing body of literature shows that capnography is the earliest indicator of respiratory distress.8,13-16 Earlier capnography systems required the patient’s trachea to be intubated, mostly limiting their use to critical care areas and the surgical suite. However, in 2004 the introduction of new technology made it possible to use capnography efficiently to monitor patients who are not intubated in general care nursing units. Nonetheless, problems have been encountered with using capnography in non-intubated patients such as compliance with use, dislodgement of devices, false or “nuisance” alarms, and restricted patient mobility. Some of these issues can be ameliorated with patient and clinician education as noted below.
In this article we describe how we determined that increased postoperative monitoring was needed; our process for determining what monitor(s) should be used; considerations that went into the cost-benefit analysis; how nursing staff was given significant process ownership; and patient outcomes achieved since introducing the increased monitoring.
St. Joseph’s/Candler Health System, Inc.
St. Joseph’s Hospital and Candler Hospital, the main facilities of St. Joseph’s/Candler Health System (SJ/C) in Savannah, Georgia are 2 of the oldest continuously operating hospitals in the United States. Patient volume is 39,064 admissions annually with 644 beds. Staff includes 407 community-based, private practice physicians, 716 nurses, and 50 pharmacists. Interaction among staff and administration is characterized by a high degree of collaboration. SJ/C has the designation of “Magnet Hospital” from the American Nurses Credentialing Center. It is a medical teaching site for the Georgia Health Science University and is affiliated with several universities for the education of pharmacists, nurses, and other health-related disciplines.
IV Infusion/PCA Safety Initiative
 An article published in 2000 by the Institute for Safe Medication Practices (ISMP) detailing potential PCA-related medication errors17 and completion of an ISMP Medication Safety Self-Assessment18 in 2001 prompted the SJ/C Medication Error Team (pharmacists, nurses, respiratory therapists, risk managers, physicians, and others) to focus intensely on the administration phase of the medication-use process and on IV medications.
An article published in 2000 by the Institute for Safe Medication Practices (ISMP) detailing potential PCA-related medication errors17 and completion of an ISMP Medication Safety Self-Assessment18 in 2001 prompted the SJ/C Medication Error Team (pharmacists, nurses, respiratory therapists, risk managers, physicians, and others) to focus intensely on the administration phase of the medication-use process and on IV medications.
In October 2002 SJ/C implemented an advanced, modular IV medication-safety system for large-volume and syringe pumps that helped avert significant IV medication administration errors.8 The need to improve PCA safety was underscored by the experience of 3 opioid-related events with serious outcomes in the preceding 2 years.
The team recognized that safe use of PCA requires both correct pump programming and monitoring of patients’ individual respiratory response to opioids.8 A 6-month beta test of new PCA and monitoring modules integrated with the existing IV safety platform was begun in June 2004. Beta testing revealed the difficulty of predicting which patients actually were high-risk, and that capnography, not pulse oximetry, provided the first indication of opioid-related respiratory depression.8 As a result, the decision was made to require a capnography module for each PCA infusion and to use a pulse oximetry module for selected patients receiving PCA analgesics who have pre-existing co-morbidities. The patient selection algorithm developed at SJ/C is illustrated in Figure 1.9
Technology
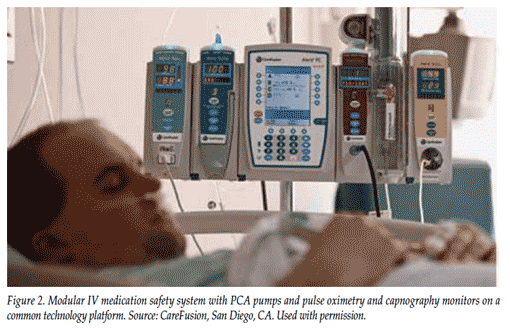
The modular intravenous (IV) medication-safety system combines large-volume, syringe, and PCA pumps with pulse oximetry and non-invasive capnography monitors on a single technology platform with a common user interface, which greatly increases ease of use and reduces possibilities for error (Figure 2). The system can be used reliably on both intubated and non-intubated patients, adult and pediatric patients, as well as patients receiving oxygen.
The non-invasive capnography system uses the filter line shown in Figure 3 to measure carbon dioxide in exhaled breaths in nose or mouth breathers. The “airway RR,” the most dependable measure of RR, is taken directly from measuring air movement in and out of the airway. In addition to RR, the system provides waveforms, exhaled EtCO2, and inhaled carbon dioxide (FiCO2) values.

An alarm, audible in the room and the hall, is generated whenever pre-established respiratory limits are exceeded. A nurse or respiratory therapist may respond to the alarm; patients may self-correct as a result of a physiologic response to the alarm, e.g. the alarm stimulates the patient to breathe during sleep apnea. The system provides up to 24 hours of PCA dosing history with corresponding time-based values from capnography and/or pulse oximetry monitoring. The trend data allow clinicians to better assess a patient’s response to PCA therapy and help provide an early warning of potential respiratory depression. When the PCA and monitoring modules are used as part of the overall system, if a patient falls below hospital-defined respiratory limits, the system’s unique “pause protocol” automatically pauses the PCA infusion and deactivates the dose-request cord.
Initially the team programmed the system to generate an alert if a patient’s RR was ≤ 10 bpm or there was “no breath” for 30 seconds. In practice, this resulted in an unexpectedly high number of “nuisance” alarms. By analyzing extensive data retained in system memory, the team determined that changing the EtCO2 parameter from 50 to 60 mmHg and resetting the RR from 10 to 6 would minimize nuisance alarms while maintaining patient safety. We confirmed these values and parameters in clinical practice as we continuously monitored patients in the clinical environment. Settings can be adjusted if necessary based on patient requirements and physician order.
Patient mobility may be limited due to the presence of the PCA pump and monitoring module(s) on an IV pole, but we did not find that the cannula caused any additional limitation. Ambulatory patients infrequently require IV PCA and most often are receiving oral pain management.
Implementation
The team recognized that for increased monitoring on the nursing units to be successful, this cultural shift needed to involve as many front-line clinicians as possible in the implementation. Physicians, nurses, pharmacists, and respiratory therapists worked together to develop policies and procedures, standardized PCA dosing forms, physician notification parameters, opioid drug libraries, routine order sets for SpO2 and EtCO2 monitoring, criteria for discontinuing monitoring and a reversal agent protocol. The use of supplemental oxygen was aligned with policies.
Having nurses involved at every step of preparation and implementation greatly increased the nursing staff’s knowledge of and willingness to use the new monitoring modules. Respiratory Care also needed to be part of the process, since respiratory therapists have an EtCO2/SpO2 knowledge base, keen clinical assessment skills, ability to intervene and resolve potential respiratory emergencies, and are available around the clock.
Education
Respiratory care and nursing with assistance from pharmacy developed a concise and basic program to introduce capnography monitoring into the general care nursing areas. Together, a clinical nurse specialist and the respiratory therapy education coordinator educated nurses and pharmacists on enhanced pain management, pulse oximetry and capnography monitoring. Education took place during staff orientation, annual competency assessments, and at the bedside. During implementation of capnography we discovered that patient education was a key component of a patient’s understanding the importance of wearing the monitoring cannula, the reason for the alarms, and response when an alarm sounded. Education materials were provided for patients and families. When educated about the benefits of capnography monitoring before going to surgery, patients are more likely to accept wearing the filter line and do very well with postoperative monitoring.
Clinical Practice
Hospital policy requires respiratory therapy to round on every PCA patient at least once every 12 hours. At each shift, the respiratory status of PCA patients is assessed by a therapist. The assessment includes an evaluation of the recorded trend analysis of RR, EtCO2 waveforms, and any pulse oximetry results. Nurses consult respiratory therapists to assist with the assessment at any time during the shift when alarms indicate potential patient respiratory distress. Early identification of respiratory depression allows respiratory therapy to intervene before a patient’s condition becomes serious, which saves time, helps increase the likelihood of a positive outcome, and allows existing staff to oversee more patients. If the nursing-respiratory team is unable to manage the patient or concludes that changes in medication, other therapy, or level of care may be necessary, the physician is contacted. The physician may change orders based on information provided and/or assess the patient at the bedside.
Results
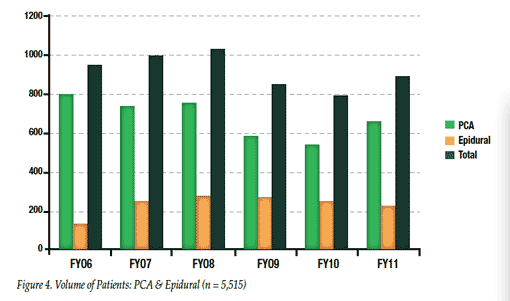
Since 2004 capnography monitoring for all patients receiving IV PCA opioid therapy has been required at SJ/C. As a result of the success of the monitoring process in patients receiving intravenous PCA, in 2006 the medical staff requested that capnography be implemented for patients receiving epidural PCA. This action was followed in subsequent years for patients receiving high-dose intermittent hydromorphone (>1 mg IV every 2 hours as needed) and for patients undergoing sedation in various types of invasive procedures. Figure 4 illustrates the distribution of PCA patient types over these years. During this period of time there have been no PCA-related respiratory events with a serious outcome, i.e. no intubations, transfers to ICU, or deaths/brain damage in more than 5,000 patients receiving IV or epidural PCA. Additionally, none have occurred with monitored patients receiving hydromorphone via PCA or intermittent IV administration or patients receiving IV procedural sedation.

The monitoring system has alerted clinicians to the potential of declining respiratory function, and appropriate interventions have been made. We quickly discovered that the EtCO2 alarm alerted nurses of respiratory depression as much as 2 hours earlier than the SpO2 alarm did, especially in patients receiving supplemental oxygen. The concentration of EtCO2 can rise even when a patient is breathing, if adequate air exchange / ventilation is not occurring, leading to CO2 narcosis. As noted above, in the 2-year period immediately preceding the implementation of the capnographic monitoring system, 3 events with serious outcomes occurred in patients receiving PCA using traditional methods of monitoring—intermittent pulse oximetry and nursing assessments. Therefore, of the available alarm methods, the concentration of EtCO2 provided the earliest indicator of opioid-induced respiratory depression.
Financial Return
There can be no adequate valuation of a life saved from preventing an adverse medication event. Nonetheless, it is important when possible to assess the potential of a new intervention to reduce the likelihood of serious outcomes and to determine the fiscal benefit of this reduction in the cost of health care. The decision in 2002 to replace the existing IV infusion pumps with “smart” modular IV safety systems, and then to add PCA, resulted in financial as well as patient safety benefits. A 5-year return on investment (ROI) was determined and previously reported.19 This analysis evaluated the incremental cost of the intravenous safety system with PCA monitoring as compared to the cost of traditional infusion pumps. Disposable costs were also included in the analysis. Continuous quality improvement data accumulated in the system identified averted medication errors and PCA monitoring interventions.
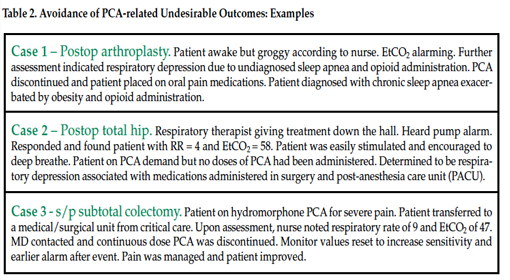
Over the 5-year period from 2002 to 2007, 558 expanded IV-safety systems helped avert 450 highest-risk IV medication errors (Table 1) and respiratory monitoring helped avert at least 35 PCA-related undesirable outcomes (Table 2), for a total of at least 471 preventable ADEs.19 In 2006 the Institute of Medicine estimated the cost of managing a serious medication-related event to be $8,750 per preventable ADE.20 These errors, if not averted, would have resulted in potential expenses to SJ/C of $3,970,296, not including potential litigation costs. Deducting the cost of averted outcomes/errors from the total purchase costs plus disposables yields a 5-year ROI of more than $2.5 million.19

Discussion
Clinical experience at SJ/C has confirmed that capnography monitoring is superior to SpO2 monitoring in the detection of opioid-induced respiratory depression. In many cases, the capnography values were the only indicator of early onset of respiratory depression. Clinician assessments have been greatly enhanced with the availability of combined dosing and respiratory monitoring trend data, particularly for EtCO2. Nurses feel more comfortable in their ability to adequately manage patients’ pain. The involvement of as many front-line clinicians as possible in the evaluation, selection and implementation of the new technology has been essential to successfully implementing and maintaining increased monitoring of patients receiving PCA therapy on the general care nursing units.
Our experience suggests that the use of capnography monitoring on ALL patients who receive PCA can reduce the incidence of adverse events from IV opioids in the postoperative setting. Selected patients should also receive continuous pulse oximetry monitoring. In the future it may be possible to more effectively utilize continuous electronic respiratory monitoring of postoperative patients. However, as the APSF has stated, maintaining the status quo while awaiting newer technology is not acceptable.
Health care providers involved in perioperative care need to fully appreciate the risk of drug-induced respiratory depression in patients receiving PCA therapy. Preventable deaths and anoxic brain injury from unrecognized opioid-related sedation and respiratory depression remain a serious and growing patient safety concern. The actions taken at SJ/C help mitigate these risks.
Based on this experience, we believe there is a pressing need for the anesthesia community to consider carefully the growing body of evidence and experience that points to capnography monitoring as providing the earliest indication of opioid-related respiratory depression.8,13-16 Continuing research and development will undoubtedly lead to even better approaches to protecting the labile patients under our care. However, there is no need to wait. Careful use of the knowledge and technology we have now can do much to help realize the vision that “No Patient Shall Be Harmed By Opioid-Induced Respiratory Depression.”
Ray R. Maddox, PharmD, FASHP
Director, Clinical Pharmacy, Research & Pulmonary Medicine
St. Joseph’s/Candler Health System, Inc
Savannah, GA
Carolyn K. Williams, BSPharm
Medication Safety Specialist
St. Joseph’s/Candler Health System, Inc
Savannah, GA
Dr. Maddox discloses that he has received speaking honoraria from both CareFusion and Oridion; however he has no financial interest in either of these companies. Ms. Williams has no financial disclosures
References
- Weinger MB. Dangers of postoperative opioids. APSF Newsletter 2006-2007;21:61, 63-67.
- Weinger MB, Lee LA. No patient shall be harmed by opioid-induced respiratory depression. APSF Newsletter 2011;26:21,26-28.
- Stoelting RK, Overdyk FJ. Conclusions and recommendations from June 08, 2011 conference on electronic monitoring strategies to detect drug-induced postoperative respiratory depression. APSF Announcements. Available at: https://dev2.apsf.org/announcements.php?id=7. Accessed December 7, 2011.
- Overdyk F, Carter R, Maddox R, et al. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient controlled analgesia. Anesth Analg 2007;105:412-18.
- Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth 2004;93:212-23.
- Walder B, Schafer M, Henzi I, et al. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol Scand 2001;45:795-804.
- Shapiro A, Zohar E, Zaslansky R, et al. The frequency and timing of respiratory depression in 1524 postoperative patients treated with systemic or neuraxial morphine.
J Clin Anesth 2005;17:537-42. - Maddox RR, Williams CK, Oglesby H, et al. Clinical experience with patient-controlled analgesia using continuous respiratory monitoring and a smart infusion system. Am J Health-Syst Pharm 2006;63:157-64.
- Maddox R, Oglesby H, Williams CK, et al. Continuous respiratory monitoring and a “smart” infusion system improve safety of patient-controlled analgesia in the postoperative period. In: Advances in Patient Safety: New Directions and Alternative Approaches, Washington: Agency for Healthcare Research and Quality; 2008;4:157-69.
- Overdyk FJ. Respiratory depression in PCA patients: what continuous respiratory monitoring has revealed. Monograph – 6th annual conference – Pain Management and Patient-Controlled Analgesia: Improving Safety and Quality of Care. San Diego, CA. Nov. 17-18, 2005
- Overdyk FJ. PCA presents serious risks. APSF Newsletter 2005;20:33.
- Practice guidelines for sedation and analgesia by non-anesthesiologists. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Anesthesiology 2002;96:1004-17.
- Miner JR, Heegaard W, Plummer D. End-tidal carbon dioxide monitoring during procedural sedation. Acad Emerg Med 2002;9:275-80.
- Hutchison, R. Capnography and respiratory depression. AJN 2008;108:35-9.
- Lightdale, J.R. Microstream capnography improves patient monitoring during moderate sedation: a randomized, controlled trail. Pediatrics 2006;117:e1170-8.
- Waugh JB. Capnography improves detection of respiratory events during procedural sedation: a meta-analysis. Available at: www.stahq.org/files/9412/9665/8167/STA_2011_Abtract_31.pdf. Accessed December 7, 2011.
- Institute for Safe Medication Practices. Design flaw predisposes Abbott Lifecare PCA Plus II pump to dangerous medication errors. ISMP safety alert; 2000 May 31. Available at: www.ismp.org/Newsletters/acutecare/archives/May00.asp. Accessed November 17, 2011.
- Smetzer JL, Vaida AJ, Cohen MR, et al. Findings from the ISMP Medication Safety Self-Assessment® for Hospitals. Jt Comm J Qual Safety 2003;29:586-97.
- Danello SH, Maddox RR, Schaack GJ. Intravenous infusion safety technology: return on investment. Hosp Pharm 2009;44:680-7.
- Committee on Identifying and Preventing Medication Errors; Institute of Medicine; Aspden P, Wolcott J, Bootman JL, et al. Preventing medication errors: quality chasm series. Washington, DC: National Academies Press; 2006.


 Issue PDF
Issue PDF