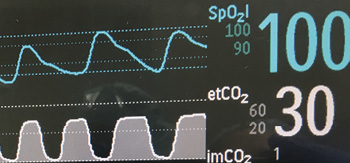
Figure 1: Depictions of continuous pulse oximetry and capography waveforms.
In 2006 and 2011, the Anesthesia Patient Safety Foundation (APSF) convened multidisciplinary conferences to address the serious patient safety issue of Opioid-Induced Ventilatory Impairment (OIVI).1Given the significance of the problem, and that no best monitor exists for detection of OIVI associated-adverse events, the consensus recommendations from the 2011 conference participants were that, until better monitors exist, continuous pulse-oximetry (preferably with centralized alarms and paging systems) should be used for monitoring patients not receiving supplemental oxygen, and ventilation monitors (capnography) are suggested for those receiving supplemental oxygen.
It is now 2017, and, in the context of the national discussion surrounding the opioid crisis, it is more relevant than ever to review the current state of monitoring for OIVI and provide updated evidence-based recommendations.
Incidence of Opioid-Induced Ventilatory Impairment
It has long been a challenge to accurately measure the incidence of OIVI and then to subsequently measure the safety advantage of a new monitoring protocol or technology. Inconsistent taxonomy for respiratory depression in the literature hinders comparative studies.2 The different definitions used as surrogates for identifying respiratory depression make determination of the actual incidence challenging. Some surrogate measures for defining respiratory depression include hypoxemia, hypopnea, hypercapnic hypoventilation, decreased respiratory rate, and minute ventilation, among others.2 Definitions used to characterize hypoxemia in the literature range from 80–94% SpO2.3 With the caveat that many different measures are used for respiratory depression, the incidence of OIVI reported ranges between 0.15% and 1.1% of all post-surgical patients.3-8 While estimates of the incidence of OIVI vary based on the definitions employed, recent studies continue to report the incidence of OIVI within this same range.2 It seems clear that the taxonomy and outcome measures for respiratory depression must be standardized so that research focusing on risk reduction can make relevant advances. In addition to determining “what to monitor,” we must decide when monitoring is needed (addressed in a companion article, We Should Focus On “When” As Well As “Whom” to Monitor for Postoperative Opioid-Induced Ventilatory Impairment) as well as the appropriate tools to reduce the incidence of OIVI.
When is Monitoring Needed
Somnolence and sedation are the most common precursors leading to OIVI.2,9 Regular monitoring by nursing staff is currently the primary means of monitoring for this phenomenon. Determining the needed frequency of nurse evaluation requires achieving a balance between minimizing patient interruption, interference with nursing workflow, and staffing expenditures. For postoperative patients, the first four hours after post-anesthesia care unit (PACU) discharge is the time period associated with the highest rates of sedation, and the first 12 hours after surgery are when over half of OIVI events occur. In addition, 75% of all OIVI events occur within the first 24 hours after surgery.2 Based on the timing of postoperative OIVI, a greater emphasis on monitoring the first 24 hours is likely to be helpful in reducing adverse events from opioids.
In 2014, The Centers for Medicare and Medicaid Services (CMS) updated their recommendations for hospital administration of opioids to include serial nursing assessments with blood pressure, temperature, pulse, respiratory rate, pain level, respiratory status, and sedation level.10 However, the optimal frequency of assessments has not been established and likely depends on a variety of factors including the type of pain, the adequacy of initial pain relief, the presence of side effects, comorbidities, and changes in clinical status. For patients receiving neuraxial opioids, the American Society of Anesthesiologists Task Force on Neuraxial Opioids and the American Society of Regional Anesthesia and Pain Medicine suggests monitoring q 1 hour for the first 12 hours, q 2 hours for the next 12 hours and q 4 hours afterward if no opioid-related complications occur.11 In contrast, a CMS-supported expert panel recommended that for any opioid administration a monitoring frequency of q 2.5 hours (to allow for documentation delays) for the first 24 hours and q 4.5 hours afterwards. However, during a survey of CMS hospitals, only 8.4% of patient encounters with IV opioid PCA met the q 2.5 hour standard and only 26.8% met the more relaxed q 4.5 hour standard.12 Because of the variation in monitoring recommendations from different organizations, different patient risk factors, different anesthetic plans, variable prescriber and nursing education regarding OIVI, and variable nurse-to-patient ratios, continuous electronic monitoring postoperatively for all patients receiving opioids is likely to simplify care and improve the detection of OIVI.
How Should Patients Be Monitored—Monitoring and Alert Systems
Regardless of the particular electronic monitoring system employed to detect OIVI, the method of alerting health care professionals when these events occur must be addressed in order to ensure an effective system. Establishing an evidence-base of monitoring alerts that are useful for detecting OIVI is a critical need. Inadequately established alert thresholds lead to alarm fatigue, patient and staff irritation, and complacency; all of which can make even the most effective monitoring system completely ineffective in achieving the desired outcome.2
Ideally, monitoring systems should use multiple parameters in concert to detect whichever indicator of respiratory depression may arise first and employ combinations of measures to accurately identify an impending event. In the past, threshold alarms have been fairly simplistic and prone to error.
Pulse oximetry is the most commonly available monitor of respiratory depression presently used in hospital systems. However, threshold alarms for pulse oximetry are often the most problematic. Setting the threshold too high leads to frequent false positives while setting it too low can result in late responses to respiratory depression. Administration of supplemental oxygen complicates the monitoring issue because it can delay detection of depressed ventilation and further impair hypoxic respiratory drive.13
Capnography used alone also has limitations. Capnography is typically qualitative instead of quantitative in non-intubated patients, thereby providing an indication of the presence of carbon dioxide during normal ventilation, relative changes in exhaled carbon dioxide, and some information about respiratory rate. However, detecting changes in CO2 values, either reduced or increased, can be problematic and inaccurate. Still, capnography can be useful as a monitor for respiratory rate since the periodic nature of CO2 exhalation and the drop to zero during inhalation provide a clear demarcation of respiratory cycling. Upper thresholds for respiratory rate can also be used with capnography to detect hyperventilation.
Combining respiratory rate with oximetry and capnography helps to provide additional information for detection of OIVI as well as other disease processes (Figure 1). Three patterns of respiratory depression resulting in unexpected death have been described by Curry et al.14 Type I is a Hyperventilation Compensated Respiratory Distress (e.g., from sepsis, pulmonary embolus, or congestive heart failure). In Type I, patients have a stable oxygen saturation initially and decreasing PaCO2 as metabolic acidosis sets in and compensatory hyperventilation begins. Rapid respiratory rate is a hallmark of this type of respiratory failure. Eventually a slow desaturation precedes a precipitous decline in SpO2 when the ventilatory response to worsening acidosis fails. Most current monitors have low respiratory rate alarms but not necessarily rapid RR alarms or the high setting detects respiratory failure too late. Type II respiratory depression is a Progressive Unidirectional Hypoventilation or CO2 narcosis event. In this case, often due to opioid or other sedative overdose, patients have a rise in PaCO2 (and EtCO2) first due to decreased minute ventilation, often while the SpO2 is still >90%. Type III respiratory depression is a Sentinel Rapid Airflow/Oxygen Saturation Reduction with Precipitous SpO2 Fall that can be observed in patients with obstructive sleep apnea. In this situation, the patient is dependent on the arousal state to maintain oxygenation. If there is arousal failure, precipitous hypoxemia develops during apnea that can lead to a sudden arrest.
There is currently no proven single monitoring system or set of alarm thresholds able to detect all respiratory patterns that result in unexpected death events. Overall sensitivity to impending events may be increased by using multiple monitors to detect patterns of change.
Newer Monitoring Technologies and Alert Algorithms
As discussed above, workforce limitations often exist for achieving the high frequency and consistent monitoring required to accurately capture adverse events and single monitor alarms are limited in their ability. Efforts are ongoing to develop and validate newer monitors with smarter alert systems.
Algorithms that combine multiple individual physiologic parameters to produce a single “superfusion” threshold may increase the sensitivity of threshold systems while still avoiding false alarms. One example is the Modified Early Warning Score (MEWS).14The MEWS is a simple additive threshold alarm that combines multiple monitors into one number for documentation and alerts. Future smart algorithms should analyze patterns of change with combinations of vital signs rather than simply adding thresholds of single monitors. These systems should predict the trajectory towards respiratory depression before an event occurs, allowing for early responses and less morbidity.
Integrated medication delivery systems and monitoring such as capnography and pulse oximetry combined with IV PCA devices allow for monitoring and response to be tied together.15 A monitor that can integrate multiple sensors and, through the use of a pattern recognition algorithm, detect early signs of respiratory depression can functionally lockout the delivery of additional opioid while alerting medical personnel.16
Respiratory rate can be measured during capnography with changes in airflow from the CO2 sampling line. However, alternative methods of detecting respiratory rate have also been evaluated. Acoustic monitoring is appealing since it can be performed without direct patient contact. This method is particularly attractive in children since maintaining a sampling line on a child can be difficult.17 However, acoustic monitoring has thus far been fraught with errors leading to alarm fatigue.18 Radar systems that monitor ventilation by mounting a sensing system in the wall or ceiling of the room are being evaluated, but are also limited by movement errors and false alarms.19
Bioimpedence is a technology that uses changes in electrical conductance of the chest obtained with surface electrodes to estimate respiratory rate, minute ventilation, tidal volume, and apnea events. Studies have shown that this type of respiratory volume monitor (RVM) can detect changes in minute ventilation and impending respiratory depression more rapidly and to a greater degree than capnography alone.20 One study found that RVM can detect the onset of respiratory depression more than 12 minutes before the onset of desaturation.21 In particular, patients receiving supplemental oxygen frequently showed signs of low minute ventilation using RVM without any desaturation alarm occurring. One of the major problems with current implementations of the bioimpedence monitors is the need for the surface electrodes placed on the patient to be physically connected to a device that analyzes the motion. In addition, non-respiratory motion such as coughing or moving can create false signals. Lastly, chest wall movement without air exchange as occurs with airway obstruction can also fool some bioimpedence devices (Table 1).17
Table 1: Pros and Cons of Continuous Electronic Monitors
| MONITOR | PARAMETERS | PROS | CONS |
| Pulse Oximetry | SpO2 HR |
|
|
| Capnography | EtCO2
RR |
|
|
| Combined Threshold (MEWS) | RR HR (SBP UOP Temp Neuro Status) |
|
|
| Integrated Delivery and Monitoring Devices | SpO2 EtCO2 RR |
|
|
| Acoustic Monitor | RR |
|
|
| Radar Monitor | RR |
|
|
| Bioimpedence | RR TV MV |
|
|
| Inductance plethysmography & audiometry | RR SpO2 Airway Patency |
|
|
| SpO2 – peripheral oxygen saturation HR – heart rate EtCO2 – end-tidal carbon dioxide RR – respiratory rate SBP – systolic blood pressure |
UOP – urine output TV – tidal volume MV – minute ventilation ICU – intensive care unit |
More complex integrated systems that combine respiratory inductance plethysmography with audiometry and pulse oximetry are very sensitive for detecting respiratory depression, but the current systems are very cumbersome, difficult for patients to wear, are subject to motion artifacts, and have similar limitations with false chest wall movements such as coughing or crying, as with other bioimpedence devices.17
Conclusions: An Ideal Future
In an ideal future, no patients will be harmed by postoperative OIVI. To achieve this goal, we will need alternative analgesics that are as effective as opioids but do not cause respiratory depression. Until then, we need to mitigate the risk of the opioid medications we currently use. This will be done through intelligent use of nursing resources combined with advanced monitoring systems that are sensitive in detecting impending respiratory events. To facilitate this future, key shareholders should help delineate a taxonomy for opioid-related adverse events including respiratory depression, with accompanying guidelines and outcome measures.
Dr. Gupta is Associate Professor of Anesthesiology at Vanderbilt University Medical Center in Nashville, TN.
Dr. Edwards is Assistant Professor of Anesthesiology, Neurological Surgery at Vanderbilt University Medical Center in Nashville, TN.
Neither author has any conflict of interest to declare as it relates to this article.
References
- Weinger M, Lee LA. No patient shall be harmed by opioid-induced respiratory depression. APSF Newsletter 2011;26:21. Available at https://dev2.apsf.org/newsletters/html/2011/fall/01_opioid.htm. Accessed December 9, 2017.
- Jungquist CR, Smith K, Nicely KLW, et al. Monitoring hospitalized adult patients for opioid-induced sedation and respiratory depression. Am J Nurs 2017;117:S27–S35.
- Sun Z, Sessler DI, Dalton JE, et al. Postoperative hypoxemia is common and persistent: a prospective blinded observational study. Anesth Analg 2015;121:709–15.
- Wheatley RG, Somerville ID, Sapsford D, et al. Postoperative hypoxaemia: comparison of extradural, i.m. and patient-controlled opioid analgesia. Br J Anaesth1990;64:267–75.
- Overdyk FJ, Carter R, Maddox RR, et al. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg 2007;105:412–8.
- Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 2010;112:226-38.
- Stites M, Surprise J, McNiel J, et al. Continuous capnography reduces the incidence of opioid-induced respiratory rescue by hospital rapid resuscitation team. J Patient Saf 2017 Jul 20. doi: 10.1097/PTS.0000000000000408. [Epub ahead of print].
- Cavalcante AN, Sprung J, Schroeder DR, et al. Multimodal analgesic therapy with gabapentin and its association with postoperative respiratory depression. Anesth Analg 2017;125:141–6.
- Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology 2015;122:659–65.
- https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-14-15.pdf. Accessed 12/15/17.
- Horlocker TT, Burton AW, Connis RT, et al. American Society of Anesthesiologists task force on neuraxial opioids. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid adminstration. Anesthesiology 2009;110:218–30.
- Jungquist CR, Correll DJ, Fleisher LA, et al. Avoiding adverse events secondary to opioid-induced respiratory depression: implications for nurse executives and patient safety. J Nurs Adm 2016;46:87–94.
- Niesters M, Mahajan RP, Aarts L, et al. High-inspired oxygen concentration further impairs opioid-induced respiratory depression. Br J Anaesth 2013;110:837–41.
- Curry JP, Lynn LA. Threshold Monitoring, Alarm fatigue, and the patterns of unexpected hospital death. APSF Newsletter 2011;26:32–5. https://dev2.apsf.org/newsletters/html/2011/fall/07_threshold.htm.
- Maddox RR, Williams CK. Clinical experience with capnography monitoring for pca patients. APSF Newsletter 2012; 26:47–50.
- Weininger S, Jaffe MB, Rausch T, et al. Capturing essential information to achieve safe interoperability. Anesth Analg 2017;124:83–94.
- Miller KM, Kim AY, Yaster M, et al. Long-term tolerability of capnography and respiratory inductance plethysmography for respiratory monitoring in pediatric patients treated with patient-controlled analgesia. Paediatric anaesthesia.2015;25:1054–9.
- Görges M, West NC, Christopher NA, et al. An ethnographic observational study to evaluate and optimize the use of respiratory acoustic monitoring in children receiving postoperative opioid infusions. Anesth Analg 2016;122:1132–40.
- van Loon K, Breteler MJM, van Wolfwinkel L, et al. Wireless non-invasive continuous respiratory monitoring with FMCW radar: a clinical validation study. J Clin Monit Comput 2016;30:797–805.
- Williams GW, George CA, Harvey BC, et al. A comparison of measurements of change in respiratory status in spontaneously breathing volunteers by the ExSpiron Noninvasive Respiratory Volume Monitor versus the Capnostream Capnometer.Anesth Analg 2017;124:120–6.
- Galvagno SM, Duke PG, Eversole DS, et al. Evaluation of respiratory volume monitoring (RVM) to detect respiratory compromise in advance of pulse oximetry and help minimize false desaturation alarms. J Trauma Acute Care Surg 2016;81:S162–70.


 Issue PDF
Issue PDF