Medication labelling can be confusing for agents that might be administered into the intrathecal or epidural space. Medications that are labeled as single-use may contain unwanted preservatives and those labeled as preservative-free may contain sulfites. Significant caution should be exercised to determine the content of medications destined for neuraxial administration.
Preservative-containing solutions are generally avoided for intrathecal administration. However, sodium bisulfites are commonly added to epinephrine-containing solutions to function as an antioxidant and increase the shelf-life for these agents.1 While much of the data describing adverse events (anaphylactoid reactions, neurotoxicity, and chronic adhesive arachnoiditis/neurologic deficits) related to intrathecal administration of these agents is historical and potentially controversial in nature, it is important to remain vigilant and aware of the impact that medication shortages and shifting suppliers may have on the constitution of many commonly utilized agents.1-5 In addition, it is important to realize that medications labeled as single-use or preservative-free may contain sulfite preservatives.

Figure 1: Single-use vial on right of image contains sulfite. Ampule on left of image is preservative-free and without sulfite.
It was recently brought to our attention that our supply of single-use epinephrine (Adrenalin®, 1 mg/mL, 1 mL single-use vial, Par Pharmaceutical, Inc., Chestnut Ridge, NY) commonly utilized to prolong neuraxial anesthesia for orthopedic procedures contains sodium bisulfite (Figure 1 – Right). Product vial labeling of “single-dose” epinephrine led to the widespread belief that this equated to “preservative-free.” While the vial label makes no mention of its inactive ingredients, the product box does provide a more thorough description of additional agents (Figure 2).6
While there were no detectable adverse events related to the intrathecal administration of this preservative-containing agent, this does highlight the importance of continued vigilance with regard to medication supplies and a good working relationship with pharmacy so that they understand the utilization of the medications being administered by anesthesia professionals.
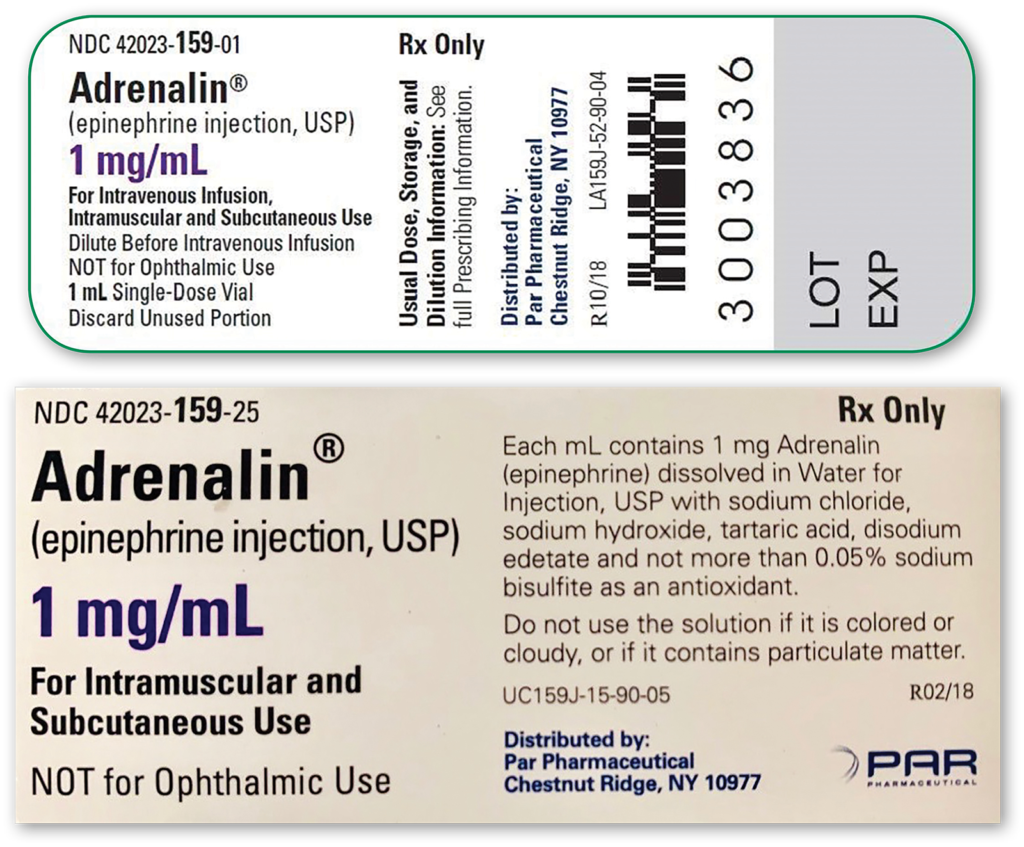
Figure 2: Manufacturing information provided for single-use (sulfite-containing) vials of epinephrine. First image provides information available on vial and second image provides more detailed information available on the product box.
To avoid the issue of preservative-containing epinephrine solutions, preservative-free ampules are now available and clearly labeled in all locations where neuraxial anesthesia is performed (Figure 1 – Left.) It is ultimately incumbent upon anesthesia professionals to ensure that products intended for neuraxial administration are specifically marked “preservative free” or, if labeled as “single-use,” are without unwanted preservatives, and collaboration with pharmacy colleagues may facilitate this process.
Kristopher M. Schroeder, MD is associate professor, Department of Anesthesiology, University of Wisconsin School of Medicine and Public Health.
Shelly B. Borden, MD is assistant professor, Department of Anesthesiology, University of Wisconsin School of Medicine and Public Health.
Trisha A. Ludwig, PharmD is pharmacy manager, UW Health at The American Center.
Elizabeth Wilson, MD, is assistant professor, Department of Anesthesiology, University of Wisconsin School of Medicine and Public Health.
Dr. Schroeder has no relevant conflicts. Dr. Shroeder is editor of ASRA News (unpaid), lecturer for Northwest Anesthesia Seminars and AudioDigest® (paid), and has an equipment grant for training purposes from Cook Medical. Drs. Borden, Ludwig, and Wilson have no pertinent conflicts of interest.
References
- Shannon JM. Neurotoxic potential of adrenaline-containing local anesthetic solutions in spinal/extradural anaes. Br J Anaesth. 2007;99:Issue eLetters Supplement.
- Rice I, Wee MYK, Thomson K. Obstetric epidurals and chronic adhesive arachnoiditis. Br J Anaesth. 2004;92:109–120.
- MacPherson RD. Pharmaceuticals for the anaesthetist. Anaesthesia. 2001;56:965–979.
- Taniguchi M, Bollen AW, Drasner K. Sodium Bisulfite. Scapegoat for chloroprocaine neurotoxicity? Anesthesiology. 2004;100:85–91.
- Khakhar MB. Preservative-containing solutions in the epidural space. Br J Anaesth. 2007;99:Issue eLetters Supplement.
- Par Pharmaceutical Inc. ADRENALIN (epinephrine injection) 1 mg/mL. 2013 [rev. January 2019]. In: Daily Med – [Internet]. Bethesda(MD): National Library of Medicine (US). Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3b7a4364-668d-4eb2-a20c-04adc35aabe4 Accessed August 8, 2019.


 Issue PDF
Issue PDF