Last updated: November 11, 2020

 |
 |
Preoperative COVID Testing:
Examples From Around the U.S. on November 11, 2020
On April 29, 2020, the ASA and APSF released “Joint Statement on Perioperative Testing for the COVID-19 Virus”. This statement currently is undergoing revision and the updated statement will be provided as soon as it is available. The recommendations of the April 29th statement include:
A population risk assessment identifying the prevalence of SARS-CoV-2 should be reviewed. When there is local or regional presence of SARS-CoV-2(14):
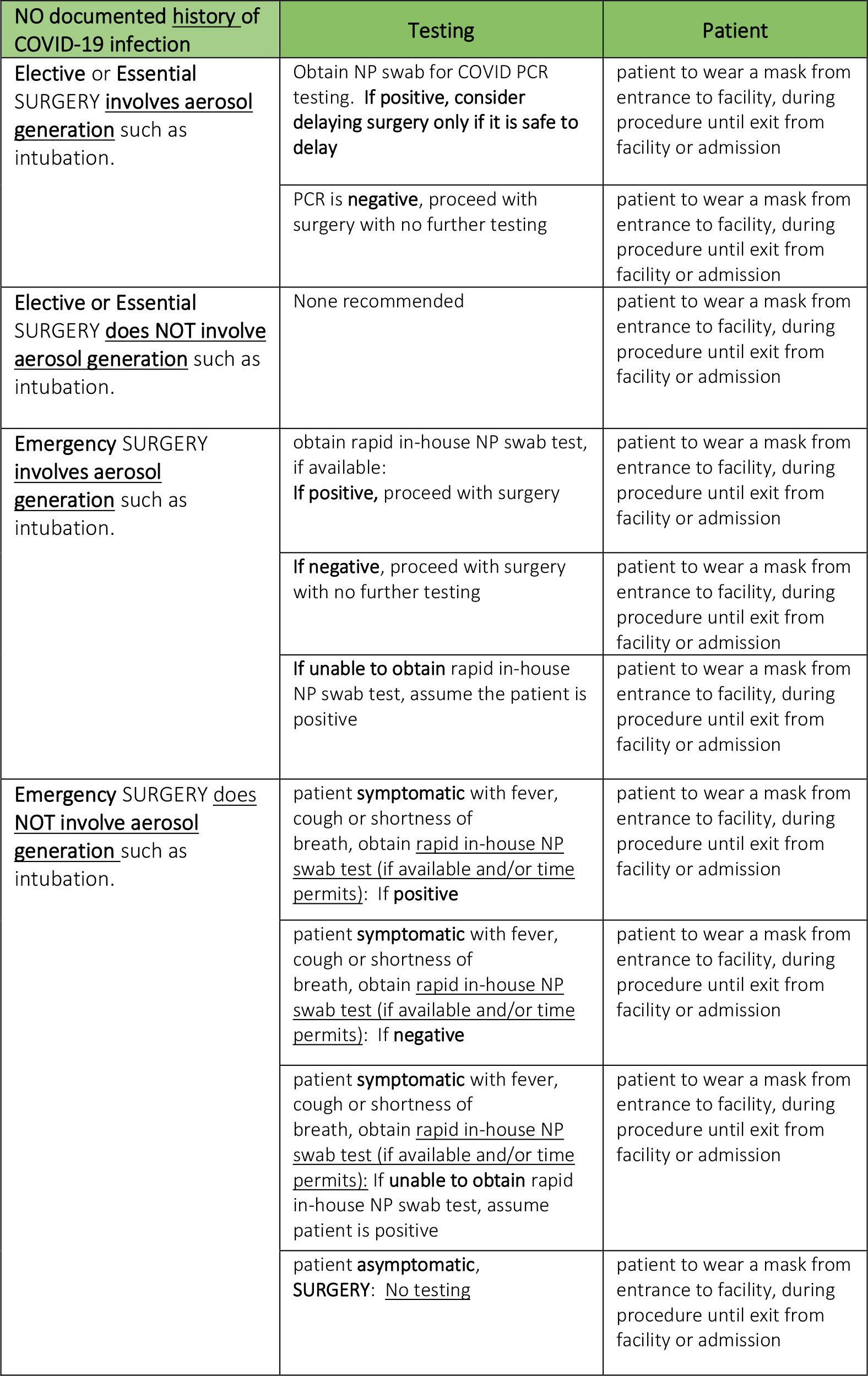
- All patients should be screened for symptoms prior to presenting to the hospital. Patients reporting symptoms should be referred for additional evaluation. All other patients should undergo nucleic acid amplification testing (including PCR tests) prior to undergoing non-emergent surgery.
- Because false-negatives may occur with testing, droplet precautions (surgical mask and eye covering) should be used by OR staff for operative cases. Before performing an aerosol- generating procedure, health care providers within the room should wear an N95 mask, eye protection, gloves and a gown.(15)
- If a patient tests positive for SARS-CoV-2, elective surgical procedures should be delayed until the patient is no longer infectious and has demonstrated recovery from COVID-19. A patient may be infectious until either:
- CDC recommended test-based strategy
- Resolution of fever without the use of fever-reducing medications; and,
- Improvement in respiratory symptoms; and,
- Negative results from two SARS-CoV-2 tests ≥ 24 hours apart
- CDC non-test-based strategy
- At least 72 hours since resolution of fever without the use of fever-reducing medications and improvement in respiratory symptoms; and,
- At least 7 days since symptoms first appeared.*
- CDC recommended test-based strategy
- Recommendations regarding the definition of sufficient recovery from the physiologic changes from SARS-CoV-2 cannot be made at this time; however, evaluation should include an assessment of the patient’s exercise capacity (metabolic equivalents or METS).
When there is little or no regional presence of SARS-CoV-2:
- All patients should be screened for symptoms before presenting to the hospital.
- Patients reporting symptoms should be referred for further evaluation.
* Please note that the CDC recently revised its recommendation on time since symptoms first appeared (above 3.b.ii.) from 7 to 10 days.
Implementation of the recommendations:
While statements and recommendations can provide guidance to local health systems for perioperative COVID testing, implementation decisions are governed by many factors, including local population prevalence of COVID-19 infection, availability and accuracy of COVID-19 tests, and others. Therefore, to assist anesthesia professionals in implementation of perioperative testing, this summary provides actual testing policies from a sample of hospitals and medical centers around the U.S. as of November 11, 2020. These facilities range from rural critical access hospitals to several of the country’s largest academic medical centers. The anesthesia practices in these facilities range from small private practices to very large group practices.
These examples are presented to provide a sense of the initial protocols used in COVID-19 perioperative testing. These protocols are continuously evolving. Those presented here come from November 11, 2020 unless otherwise noted, and many will rapidly evolve in future weeks as testing availability, accuracy, cost-effectiveness, and practice efficiency also evolve.
The participating health systems will be asked to provide any updated protocols every second week during the next two months so that this summary can be updated regularly. Please check the summary date to ensure you understand when this set of updates was made.
QUICKLINKS to INSTITUTIONS:
Chester County Hospital, a part of the Penn Medicine
Chester County Hospital is a 248-bed inpatient complex in West Chester, PA. These are the guidelines of the hospital for the care of COVID patients in the perioperative unit as of November 11, 2020.
Pre-Procedure COVID Testing:
- All procedural patients will be screened for exposure and symptoms upon registering according to the following guidelines:
- Outpatients:
- Previous COVID diagnosis: If diagnosed positive within the last 14 days, treat as positive.
- Previous COVID diagnosis: If diagnosed positive within the last 15-28 days, obtain 2 negative swab results (1 day apart) to be treated as negative, otherwise treat as positive.
- Group Homes/Congregate Settings (i.e. Skilled Nursing Facilities): Testing required 2 days prior to procedure but are placed on COVID precautions regardless of result.
- ALL OTHER OUTPATIENTS: Testing required within 2 days prior to procedure.
- Inpatients:
- COVID Positive: No testing necessary (IP will manage the need for repeat testing per protocol)
- COVID Risk: No testing necessary, treated as positive (IP will manage the need for repeat testing per protocol)
- Non-COVID: Testing accepted within the last 7 days
- Group Homes/Congregate Settings (i.e. Skilled Nursing Facilities): Must be tested upon admission.
- Outpatients:
- All outpatients/24 hour/AM Admits will be notified to come in for testing the 2 days before their scheduled procedure.
- The patient will report to the Employee Resource Center for testing.
- Positive results will be reported to the surgeon to determine whether the case could be postponed.
- Positive results will be communicated to the patient.
Pre-Procedure COVID Considerations:
- Patients awaiting COVID rapid results (45 minutes) will not be transported to the Operating Room until results are obtained and communicated (exception for ruptured AAA).
- The nurse pulling the patient onto the Snap Board must notify PACU of the patients COVID status.
- COVID patients will be assigned to a private room in ACC and will be brought directly from ACC to the OR.
- PPE requirements for COVID patients in ACC will observe droplet-based precautions, which include gown, gloves, standard mask, and eye protection. The patient should be given a hospital-issued mask immediately upon arrival.
- (Inpatients Only): Prior to being brought to the OR, if the COVID patient is already in a negative pressure inpatient room, they will be intubated in that room prior to transport; consult anesthesia team
Hawaii
During this pandemic, Hawaii paused elective surgery in April/early May. When we restarted surgery, Hawaii’s COVID Rt was 0.71—the lowest in the U.S. at the time— and our prevalence rate was very low. Since then, the state rose to the highest Rt rate in the country, our hospitals and ICUs burgeoned at capacity+, and our 7-day positivity test rates were in the double digits, with some vulnerable communities reporting test positivity rates as high as 29%. In late August, we dropped back to the lowest Rt and we currently have an Rt just below 1.0. As of October 1, we have a statewide 7-day case rate of 7.3/100,000 (Honolulu 9.6/100,000) and a statewide 7-day positivity rate of 2.9% (Honolulu 4.0%).
Our preoperative testing strategy has evolved since our initial report to APSF in mid May. We continue to review the local contagiousness of the disease, COVID test positivity rate (current proxy for prevalence), rate of new cases/active cases in relation to our health system capacity, burden/burn rate of PPE, capacity and turnaround time for testing, and specificity/sensitivity of our tests as factors to optimally design the continuously evolving recommendations for both outpatient and inpatient surgery. These algorithms guide our new discipline to sustain the state and our medical community through future waves.
This summary describes two community hospital systems and their approaches to preoperative COVID testing. Hospital A is a four-hospital system with a free-standing ambulatory surgery center. Hospital B is another four-hospital system. Both have presences on neighboring islands—this geography complicates patient access to testing.
What is similar between the two community hospital systems and their preoperative COVID testing protocols:
- Testing all elective surgeries and procedures with RT-PCR antigen test (including full term at 38-39 weeks, elective labor inductions and elective C-sections, endoscopy, cardiology cath lab, TEE, interventional radiology, pulmonary PFT and Sleep Lab ).
- Utilizing internal laboratory testing with known high levels of sensitivity and designated testing centers with known reliability.
- Results must be available prior to surgery to avoid cancellation
What is different between the hospitals:
- Example B has a list of serial procedures that do not require repeat testing (e.g. serial ECTs, serial wound debridements, staged procedures, or serial opthalmologic surgeries) allow for a test to be valid for 14 days.
- Responsibility of ordering test (Example A lies with the surgeon/proceduralist; Example B lies with a centralized presurgical center)
- < 72 hour window vs. < 7 day window. Both systems reviewed their workflow to create the most disciplined, reliable results.
- Importantly, both hospitals have eliminated a 2 negative test strategy to proceed with surgery in a patient who has tested COVID+. Revised algorithms support CDC’s symptom-based vs test-based removal of isolation/ infectious status for decision-making. This is one area of evolution, as we continue to learn about persistent shedding and the potential for reinfection.
- Example A allows for an elective procedure 1 month following the positive test if patient is otherwise well. After 3 months, the patient must retest and clear before undergoing elective surgery
- Example B advises to wait 6 weeks following a positive test. If there is a plan to proceed with elective surgery within the 6 weeks, the patient must meet CDC symptom-based isolation clearance AND be asymptomatic with low risk behavior. Retest is required 6 weeks after a positive test.
Specific recent updated changes with Example B:
- Roche Coba SARS COV-2 for asymptomatic screening of elective pre-procedural/aerosol-generating procedures. Abbott ID NOW COVID-19 (TAT ~ 15 minutes) for patients requiring urgent/emergent (e.g. trauma) surgeries and aerosolized generating procedures.
- (In the algorithm submitted, note all patients are being double tested with both the Roche Cova and Abbott ID NOW. This is a current temporary cautionary measure due to rare false-negative results)
- Swab testing site has been set up in one of the physician office buildings to perform same day swab testing (allowing patients to go from surgeon’s office to swab testing site conveniently).
Similar to reports across the country, hospitals in our state have experienced asymptomatic
- Patients with false-negative results
- Patients with real-negative results upon admission converting to real-positive results during the admission
- Point prevalence testing detecting previously unknown COVID positive patients in hospital.
- Employees becoming infected from source exposures in break rooms and after-hour gatherings.
To limit unintended consequences of clinician illness, quarantine, isolation, and the potential impact of significant staff absence management, leadership and peers continue to caution staff not to let their guard down. Efforts focus on designing workflows that enable exercising reliable prevention measures at work and outside of work. Anesthesia/airway teams are advised to wear N-95 and face-shields during all airway encounters.
What is clear is that these algorithms are constantly evolving and subject to change. Both hospital systems have town hall/communications several times a week to keep everyone current with ongoing efforts to ensure patients and staff are safe.
Example A:
System requires testing 72 hours prior to procedure, surgeon/proceduralist orders PCR test.
Example B:
Hospital System with workflow for all outpatient procedural/surgeries routed through Pre-Surgery Center to screen and order PCR testing
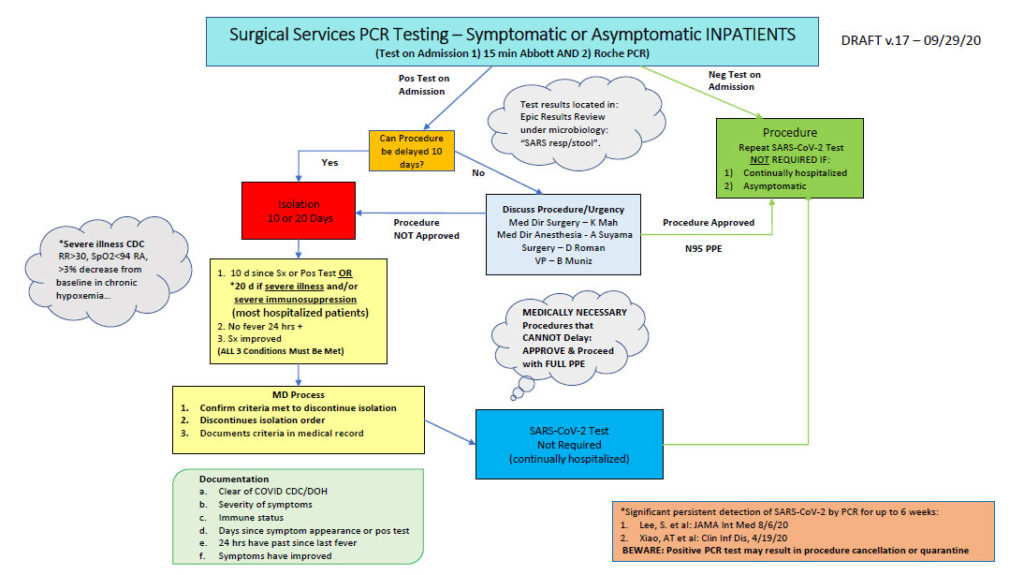
Courtesy, Beryl Muniz, RN, Vice President of Patient Care for Surgical Services and Emergency/Trauma Services (additional thanks to Scott Gallacher, MD, Kathy Mah, MD and Alan Suyama, MD)
Houston, Texas
A large private practice anesthesia group provides professional services at a dominant tertiary healthcare system in Houston, Texas. The main facility is over 1000 beds and located in the Texas Medical Center. The healthcare system also includes 5 other satellite hospitals located within the city and surrounding community. All of the system hospitals are currently using the perioperative testing protocol outlined below.
The biggest change in the Houston protocol this past summer is, instead of using a nasopharyngeal swab, we are using nasal swabs for asymptomatic patient COVID-19 testing. The nasal swab, which collects the sample in the front part of both nostrils, is found by many to be more comfortable than the nasal pharyngeal swab. The nasal swab collections are used for asymptomatic non COVID-19 patients who are admitted to our hospitals as well as presurgery/preprocedure screenings.
COVID-19 Testing
- The physician orders the test via electronic medical record or fax.
- The physician schedules the procedure or surgery with the appropriate department
- The patient is contacted by the hospital to schedule the COVID-19 test.
- There are no interviews to screen patients for symptoms or to determine if they have had recent exposure to potentially infected persons.
- COVID-19 testing is required for ALL patients undergoing any surgery, procedure, or MRI which will require intubation. Testing is also required for all patients undergoing transesophageal echocardiography.
- The test is a COVID-19 Polymerase Chain Reaction (PCR) from a nasopharyngeal swab sample.
- Patients must have testing done at least 5 business days and no greater than 8 business days prior to the surgery/procedure date.
- Patients are asked to quarantine after testing until the day of surgery or procedure.
- If the preoperative COVID-19 test is positive, elective surgery is postponed.
- COVID-19 testing is also available for symptomatic patients who do not have a scheduled procedure.
Mayo Clinic, Rochester, Minnesota
The Mayo Clinic is a large health care system. There are three major regions: the upper Midwest, northeast Florida, and Arizona. Of these, the upper Midwest is comprised of one large tertiary medical center and 15 additional hospitals/facilities within 200 miles of Rochester, MN. For simplicity, this document will provide information on only those facilities within the upper Midwest. The upper Midwest facilities range in size from critical access hospitals in small, rural cities to a large complex in Rochester that provides anesthetic care for more than 400 inpatients and outpatients, including non-operating room procedures, daily.
The screening process for preoperative and pre-procedural patients is a multi-step process. All patients for whom this process is initiated should undergo all steps.
Below is the Pre-Surgical Screening Process:
Pre-Surgical Screening Period
STEP 1: Telephone Interview, Testing Instructions
Patients identified after the Patient Selection process will be contacted by the surgical team (see Appendix for scripting) for a telephone-based interview. As part of this process the patient will be asked several brief questions:
Does the patient, anyone in the household, or anyone to whom they have had prolonged exposure in the past 5 days have (any of the following)?
- Fever ≥ 37.8 C (100.0 F) last 48 hours
- Symptoms include new: cough or shortness of breath, sore throat, diarrhea, nausea, vomiting, respiratory distress, chills, muscle aches, repeated shaking with chills, headache, loss of smell, or change or loss of taste sensation
- Does the patient or visitor have close contact with a person under quarantine or isolation, or is a LABORATORY CONFIRMED case of COVID-19? Close contact as defined by the CDC as: Being within approximately 6 feet of a COVID-19 patient for more than 5 minutes or having direct contact with infectious secretions of a COVID-19 case (e.g. being coughed on)
- Have you been tested for COVID-19 with a positive or pending result?
A positive answer to any of these questions will result in the patient being considered interview-positive. This knowledge will be communicated to the clinical team responsible for the operation and the patient will be placed back on the deferred list and transferred to the COVID Nurse Line.
Even if telephone interview responses are negative, patients will be referred for COVID screening tests (see STEP 2 below).
If patient interview is negative, proceed with the following steps:
- PCR Testing
- The foundation of our screening is a COVID-19 PCR swab obtained from the nasopharynx. Our best practice should be to obtain the test within 2-3 days of surgery.
- Except for emergency cases, all patients must have a negative PCR before proceeding to the operating room (OR).
- It is the responsibility of the primary surgical service to order and monitor the results of pre-surgical COVID-19 PCR testing. Patients with positive or pending PCR results should be informed to not report to the hospital, as their procedure will need to be rescheduled.
- Patients requiring PCR testing for other procedures or pre-operative studies (e.g. pulmonary function tests, bronchoscopy, endoscopy) do not need to be retested again prior to surgery if the negative PCR was within 5 days.
- PCR Retesting
- Patients with COVID-19 infection may continue to have a positive PCR for SARS-CoV-2 RNA for weeks after resolution of the infection. Based on studies using cell culture and contact tracing, a positive PCR does not appear to reflect the presence of infectious virus among patients who have recovered from their infection. There have been no documented cases of clinically recovered people with persistent viral RNA who have transmitted SARS-CoV-2 to others after 30 days have passed since their infection onset.
- PCR testing should not be repeated for 90 days from the date of initial COVID-19 diagnosis in most cases. This includes prior to procedures or surgery.
- Patients who develop new COVID-19 symptoms should continue to be tested according to symptomatic testing algorithms.
- For patients who have recovered from COVID-19, and are less than 90 days from the date of diagnosis, there is no restriction on care, procedures or surgery, and retesting by PCR is not necessary unless the patient has new COVID-19 symptoms.
McLaren – Greater Lansing (Michigan)
McLaren Greater Lansing (formerly Ingham Regional Medical Center), a teaching hospital located in Lansing, Michigan, is a subsidiary of the McLaren Health Care Corporation. It is affiliated with both the College of Human Medicine and the College of Osteopathic Medicine of Michigan State University.
At McLaren Greater Lansing, Covid-19 testing is a request initiated by the physician performing the procedure. They are no longer testing every patient prior to surgery. If a test is requested, a preoperative nurse will contact the patient to schedule the PCR test (Roche) 5-7 days prior to surgery. The patient is instructed:
- Monitor your temperature twice a day for 7 days before your procedure
- Practice social distancing for up to 7 days prior to your procedure
- If possible, wear a face covering when in public spaces with crowds for up to 7 days prior to your procedure
- Continue to wash your hands or use hand sanitizer frequently
- Reach out immediately to your provider if any symptoms arise
Their preoperative nurse will contact the patient again 1 day before the procedure with screening questions and the test results, if ordered. The patient is instructed not to call the lab for results, all preoperative notifications are handled by their preop nursing staff.
Medical University of South Carolina
Pre-procedural Testing Principles
- The following guidance addresses the question: “Is my patient at-risk for transmitting COVID to the care team?”. The guidance does not address the question: “Do the expected benefits of the planned procedure outweigh the anticipated risks for my patient?” The latter can only be determined by thoughtful, joint review of the full clinical picture by surgeon, anesthesiologist, and patient.
- If a patient was COVID PCR(+) at any time in the preceding 180 days, and otherwise meets the clearance criteria in the following slides, pre-procedural testing is not warranted.
- Patients who are COVID antibody(+) at the time of a planned procedure also should not undergo additional pre-procedural testing.
- For outpatients, COVID nasopharygeal PCR testing within 4 days of the procedure
- For inpatients, COVID PCR testing within 7 days of the procedure
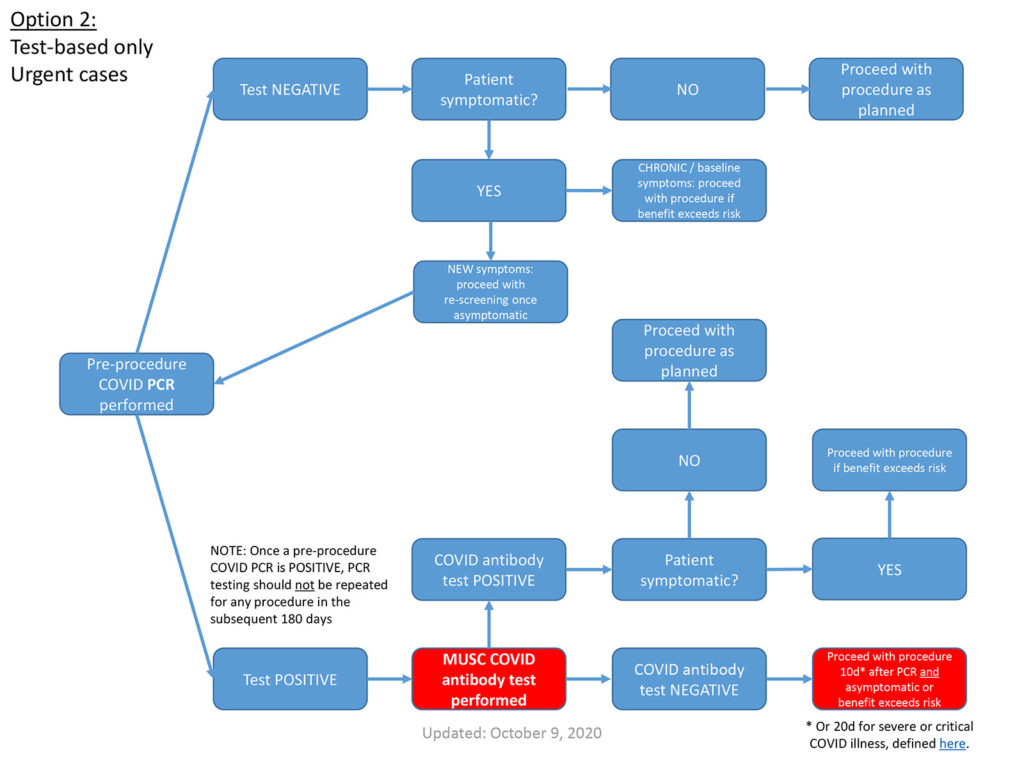
* Or 20d for severe or critical COVID illness, defined here.
Mercy Hospital, St. Louis, Missouri
Mercy, is a large health system located in the lower Midwest that includes more than 40 acute care, managed and specialty (heart, children’s, orthopedic and rehab) hospitals, 900 physician practices and outpatient facilities, 45,000 co-workers and 2,400 Mercy Clinic physicians in Arkansas, Kansas, Missouri and Oklahoma. Mercy also has clinics, outpatient services and outreach ministries in Arkansas, Louisiana, Mississippi and Texas. In addition, Mercy’s IT division, Mercy Technology Services, and Mercy Virtual commercially serve providers and patients from coast to coast.
Perioperative Guidance [Download PDF Version]
This document provides guidance for patients scheduled for surgery. The document will be updated as new guidance becomes available.
Basic Pre-Surgical Requirements:
- Testing:
- Non-COVID Patients: Pre-surgical testing is to be completed for
- Elective or essential surgeries that may require intubation or an aerosol generating procedure, ideally within 48-72 hours in advance of the surgery. Each facility should pre-identify surgical cases meeting this criteria.
- In situations where testing is not available or outside this testing window, the decision may be made to proceed with surgery without testing when combined agreement with anesthesia and surgeon is obtained. If both providers agree to proceed without testing, follow guidance in table (page 4 and 5) for PPE and room management.
- Facilities will need to identify local sites for patients to go for testing.
- PCR testing will be performed using Quest 48-hour turnaround time (TAT) testing or designated facility platform using batch methodology (in-house rapid test reserved for COVID PUI).
- COVID Positive Patients Asymptomatic – Pre-surgical testing is NOT needed if it is within 90 days from symptom onset and patient remains asymptomatic. (CDC: studies that have attempted to perform viral culture on patients beyond day 9 of illness have not been able to demonstrate the virus is infectious).
- Non-COVID Patients: Pre-surgical testing is to be completed for
- Elective surgery should be postponed on a patient:
- With a medical history of COVID-19 until patient has recovered from illness and has been released from isolation.
- Isolation duration criteria: In general, at least 10 days after symptom onset for immunocompetent patients with mild-moderate illness and 20 days for severe/critical illness or severely immunocompromised** AND patient must also remain afebrile for 24 hours without fever reducing medications AND have improvement in symptoms.
- Scheduling or rescheduling the patient for surgery should only be considered after the patient has met the 10-day or 20-day time period based on the severity of illness described above. Once the time period has been met, the patient’s condition must be assessed to verify they are fever free and have improvement in symptoms.
- Who has any symptoms concerning for COVID-19 – Symptomatic patients should be evaluated, and elective surgeries postponed until acute illness has resolved, if possible.
- With a medical history of COVID-19 until patient has recovered from illness and has been released from isolation.
- Patients are instructed to limit time spent out in the community (self-quarantine) prior to surgery to avoid any potential for COVID-19 exposure.
NorthShore University HealthSystem, Illinois
NorthShore University HealthSystem is a five hospital system largely located in the north shore of Chicago. Our largest hospital is located in Evanston, IL, and serves as the tertiary care center for both two hospitals in Lake County (Highland Park Hospital and Glenbrook Hospital) and another hospital in Cook County (Swedish Covenant). NorthShore University HealthSystem provides anesthetic care for more 70,000 patients, including non-operating room procedures, annually. University of Chicago is an academic affiliate of NorthShore and provides a complement of anesthesia residents for a variety of clinical care experiences. In addition, our system provides clinical education for SRNAs. Lastly, we have over 75 CRNAs, and over 60 anesthesiologists covering the 5 hospital system.
The NorthShore University HealthSystem adult preoperative testing protocol is continuously evolving as we learn more about COVID testing and its impact on outcomes. As of November 11, 2020, the protocol entails the following processes and algorithm: See appendices for detailed information on the process of COVID-19 preoperative testing and management.
- MEMO – Elective Procedure COVID-19 Testing Memo [PDF]
- APPENDIX A – Pre-Surgical Procedure COVID Testing Scripting [PDF]
- APPENDIX B – COVID Tent Testing Messaging and Handouts [PDF]
- APPENDIX C – ALGORITHM Elective Procedure COVID-19 Testing Flow Chart [PDF]

New York University, New York
NYU is a major academic medical center in the heart of New York City. The center has in-hospital and outpatient facilities, but has developed one policy for preoperative COVID testing of all patients for COVID status. The following is a component of the preoperative process we currently use for care of patients, and as we are constantly reassessing our process, it should not be construed as a recommendation for others to use.
Preadmission and Day Before Procedure Call Process
The patient is screened for COVID related issues including symptoms and fever, however not all patients are reached on preoperative or preadmission testing calls. If symptomatic or issues related to COVID there is a joint collaboration among the preoperative team, the surgical team and patient to follow evidence based institutional guidelines.
Preop Covid Testing Policy for Patients undergoing Surgery or a Procedure
- Covid PCR 19 testing must be performed within 5 days of a procedure or surgery
- PCR 19 testing may be performed the day of the procedure in preop area but may take 2hr to be resulted
- OB patients admitted to labor delivery must have PCR testing within 7 days of admission
Screening PCR prior to a procedure or surgery is not required in the following asymptomatic patients
- Continuously hospitalized patients who underwent PCR 19 testing on admission and have no history of exposure to Covid during hospitalization
- Ambulatory patients having sequential non-elective procedures such as radiation therapy or ECT over multiple days, unless 14 days have elapsed since their last negative Covid PCR test
- Patients with a positive Covid -19 PCR within 90 days prior to the procedure
Day of Surgery
- The patient is screened for COVID related issues and symptoms and their temperature is obtained
- For asymptomatic patients with PCR COVID Positive test – if possible, defer surgery for 10 days without repeat testing
Of note, if 90 days has elapsed since an asymptomatic positive PCR 19 test, the patient should be retested. If the PCR 19 is again positive, the surgery should be delayed 10 days. Serology and antigen assays are not accepted as screening test.
Stanford Hospital, Palo Alto, CA
Stanford University Medical Center is a medical complex which includes Stanford Health Care and Stanford Children’s Health. Stanford Health Care provides both general acute care services and tertiary medical care for patients locally, nationally and internationally. The hospital plays a key role in the training of physicians and other medical professionals. It provides a clinical environment for the medical school’s researchers as they study ways to translate new knowledge into effective patient care. Full-time Stanford faculty and community physicians make up the hospital medical staff. Stanford Hospital is home to a Level I trauma center.
Tulane University Medical Center
The Tulane Medical Center consists of two facilities, which include our Downtown Campus and our Lakeside Campus. For simplicity, other affiliated medical centers are excluded. The Downtown Campus is a 235 bed facility in the City of New Orleans and houses several intensive care units and a wide array of surgical and procedural services. The Downtown Campus serves as the main practice location and teaching facility for the Tulane Medical School faculty. The Tulane Lakeside Campus is 120 bed facility and houses the obstetric unit as well as surgical suites serving as the primary location for orthopedic services. The Lakeside Campus is in Metairie, a suburb of New Orleans, less than 10 miles from the Downtown Campus.
Pre-Operative COVID-19 Screening Protocol:
- Initial Screening:
- ALL patients scheduled for elective surgery must have a PAT visit in person or alternatively, a phone interview or tele-visit with a PAT NP if the patient cannot come to PAT for evaluation.
- On the day of surgery all patients must comply with temperature screening upon entering the facility. A COVID-19 symptom questionnaire must be completed. Patients will minimize any possible exposure and self-isolate if necessary prior their surgery.
- All in-patients requiring surgery must have a SARS-nCoV-2 test performed within 48 hours of the scheduled procedure. For most patients this will be done using the Abbott ID NOW™ test the morning of surgery. In patients may satisfy the requirement with either the Abbott test or the Roche Diagnostics Cobas® SARS-CoV-2 test.
- Positive Results: If positive COVID-19 testing or positive symptom survey, serious consideration must be given to canceling the procedure. A Multidisciplinary panel may be convened to discuss the risk/benefit ratio of proceeding with surgery if required. Possible scenarios include:
- If positive symptom survey and negative COVID-19 testing: re-test for COVID-19 and/or ruling out other infections, i.e. influenza.
- If positive COVID-19 testing and negative symptom survey: postpone the procedure and, if needed, hold a meeting with the Multidisciplinary Board for a risk/benefit evaluation to consider proceeding with the surgery or procedure.
- If positive symptom survey and COVID-19 test: Postpone the procedure and, if needed, consult with the Multidisciplinary Board meeting to perform risk/benefit evaluation if the procedure cannot be postponed.
- If negative screening and negative ABBOTT test à proceed with the procedure
- Multidisciplinary Panel: Can be convened to decide concern performing a procedure on a patient with a positive COVID-19 test and/or positive symptom survey. The Multidisciplinary Panel will consist of a surgical team member, the Director of Surgical Services, an anesthesiologist, a critical care specialist, and an infectious disease specialist. The Multidisciplinary Panel will assess the risk/benefit of proceeding to surgery in the event that surgery should no longer be postponed. Retesting may be necessary.
- PPE Protocol Adherence: The PAT and OPS staff will wear level 3 masks throughout the patient’s care visit even if the test is negative. During testing in PAT, the staff will adhere to the PPE requirements (N95 Mask, gown, and face shield). If the test is positive all members will wear an N95 with a face shield.
Pre-Operative COVID-19 Symptom Survey
The survey must be completed within:
- 5-7 days, and not greater than 9 days pre-operatively
- Day of surgery (or day prior, surgeon’s discretion)
The questionnaire must cover typical symptoms1 of COVID-19 infection:
- How are you feeling?
- Have you had any recent:
- Fevers/chills?
- Cough?
- Shortness of breath?
- Muscle aches/pains?
- Diarrhea?
- Abdominal pain, nausea, vomiting?
- Loss/decrease in taste and/or smell?
Epidemiological Risk Assessment:
- Have you recently been hospitalized for COVID-19 / Coronavirus or a flu-like illness?
If yes, when? - Have you recently been diagnosed with COVID-19 / Coronavirus? If yes, when?
- Have you recently been tested for COVID-19 / Coronavirus? If yes, when?
- Do you believe that you have had close contact with a person or family member who currently has or has recently had COVID-19 / Coronavirus?
If yes, when? In what context? - Do you live with anyone who currently has or has recently had (last 14 days) fever/chills, cough, SOB, muscle aches/pains, or flu-like symptoms?
- Do you have any close contacts or family members who are currently in the hospital or were recently in the hospital for COVID-19 / Coronavirus or a flu-like illness?
If yes, assess if there was recent close contact with this person. - Do you have a home thermometer?
Veterans Affairs Palo Alto Health System
Pre-procedural COVID-19 testing
Procedure type:
- COVID-19 testing is expected for all veterans undergoing procedure/surgery at Palo Alto VA that requires anesthesia (GA, MAC).
- Procedures that do not require anesthesia will proceed with the existing selective testing protocol [based on risk of procedure and patient risk].
Timing:
- Testing should be done within 3 days of procedure. Efforts should be made to test patients at the most convenient testing site for the patient.
- Exceptions: Due to potential constraints of testing facility availability, testing turnaround times, geographical challenges, and potential patient hardship, if there is a negative test done within 5 days of the procedure, then no repeat testing is required prior to the procedure provided rigorous adherence to COVID-19 screening questionnaire on the day of the procedure.
- Note: A negative test does not eliminate the risk of COVID-19 infection, due to the 14-day incubation period and low test sensitivity, early in the infection. To minimize risk, patients should be advised to self-quarantine prior to the procedure, if possible at minimum from the date of the test, but optimally 14 days prior to the procedure.