Unraveling of the reported unexplained appearance of carbon monoxide during general anesthesia, which is presumably related to an interaction of a potent volatile anesthetic and the carbon dioxide absorbent, has begun through a series of experiments at the University of California, San Francisco (UCSF) and is revealed here for the first time.
Two articles in the APSF Newsletter (Summer, 1994) indicate that carbon monoxide toxicity represents a potential hazard of the administration of modem halogenated volatile anesthetics, including the newest of these, desflurane.(1,2) Headlines of these reports suggest that the bases for such toxicity and the relationship to the choice of anesthetic or absorbent are unclear. Questions have been raised. Why are the reports of measurable levels of carbon monoxide rare? Why do the cases appear to occur in patients anesthetized on Monday or after a period of non-use of the anesthetic equipment for two days? Why does the development of carbon monoxide not consistently correlate with the duration of use of absorbent? (3) Why have most of the past reports been associated with enflurane, with a new report associated with desflurane?
Results from preliminary studies conducted at UCSF supply answers to these questions. The results suggest guidelines that should prevent the production of carbon monoxide. Although no report has indicated that patient harm has resulted from the production of carbon monoxide during general anesthesia, avoidance of such a risk would seem prudent and in the best interest of patient safety.
Methods and Materials
We used standard commercially-available soda lime (Sodasorb) and Baralyme, and also soda lime and Baralyme we dried to various degrees for purposes of the experiments. We directed a flow of 12.5 mL/min of desflurane, enflurane, halothane, isoflurane, and sevoflurane, each at a concentration of what would be approximately 0.8-1.0 MAC or approximately 2 MAC, through tubes containing 21-25 g of these absorbents. The tubes (in duplicate for each determination) were placed in a water bath to control temperature. Using gas chromatography, we measured any carbon monoxide issuing from tubes (limits of detection approximately 1-10 ppm). The chromatograph employed a molecular sieve and a thermal conductivity detector. Similarly, using flame ionization detection and a column containing 10%/SF96 on Chromosorb WHP, we determined the inflow and outflow concentrations of the volatile anesthetic.
Results
Degradation of Anesthetics by Standard Absorbents: No appreciable production of carbon monoxide resulted from the action of standard (with normal moisture content referred to as “wet”) absorbents on any of the anesthetics at temperatures of up to 45’C, although sevoflurane (but not other volatile anesthetics) degraded in the presence of both absorbents. Standard (wet) carbon dioxide absorbent granules contain 13% water (in Baralyme) to 15% water (in soda lime).
Effect of Temperature and Anesthetic on Carbon Monoxide Production from Dry and Partially Dry Absorbents: In contrast to the effect of standard (Wet) absorbents, completely dry (no water) soda lime and Baralyme and partially dry soda lime and Baralyme produced carbon monoxide when exposed to all the volatile anesthetics, and did so to higher and/or more prolonged peak levels as the temperature of the reaction was increased. Although carbon monoxide resulted from exposure to all the anesthetics, the amounts produced with halothane and sevoflurane were small. In contrast, substantial amounts could be produced with all three anesthetics containing the CHF2 Moiety. Among these three anesthetics, the highest peak level of carbon monoxide was produced with desflurane, followed next by enflurane and then by isoflurane.
For example:

Dry Baralyme at 45’C produced still higher peak levels:
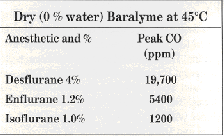
These peak levels were not sustained, progressively decreasing with continuing flow of anesthetic through absorbent. Thus, after 4 hours (240 min) of flow through dry Baralyme, the respective values with dry Baralyme were 2,070 ppm, 1,050 ppm, and 1,250 ppm.
Partial wetting of the absorbents (retention of some water but still not the normal amount) dramatically decreased production of carbon monoxide. For example, soda lime containing 1.4% water (i.e., one-tenth the normal water content) at 45’C produced much lower peak levels than dry soda lime: 5.0% desflurane 230 ppm; 1.2% enflurane 230 ppm; and 1.0% isoflurane 110 ppm. With soda lime, at a 4.8% water content, no carbon monoxide was produced. Achieving a similar effect with Baralyme required more wetting. Baralyme containing 1.6% water still produced high peak levels of carbon monoxide at 45’C (4.0% desflurane 14,800 ppm, 1.2% enflurane 4,400 ppm; and 1.0% isoflurane 980 ppm), but Baralyme containing 4.7% water produced levels roughly equivalent to those produced by soda lime containing 1.4% water. With Baralyme containing 9.5% water (still less than the normal fraction), no carbon monoxide was produced by the interaction of Baralyme and the volatile anesthetics. As with completely dry absorbent, peak levels were not sustained. For example, after 240 min of flow through Baralyme containing 1.6% water, the levels were: desflurane 1,500 ppm, enflurane -1,400 ppm; and isoflurane 1,000 ppm.
The above data for peak carbon monoxide levels exaggerate both the sense of the dose of carbon monoxide delivered and the apparent differences among the three anesthetics. The exaggeration of the impact of the dose results because of the rapid decay from the peak and because higher peak levels also were associated with an even more rapid decay in carbon monoxide production. For example, the above-noted peak values for 1.6% water in Baralyme differ by a factor of 15 (desflurane giving a value 15 times greater than that for isoflurane). However, the average values for a four-hour period of delivery differ by a factor of 5 (desflurane 4,700 ppm, enflurane 3,400 ppm, and isoflurane 900 ppm).
Relationship of Anesthetic Degradation to Carbon Monoxide Production from Desflurane, Enflurane and Isoflurane: The peak levels of production of carbon monoxide coincided with the total or near-total destruction of each anesthetic by a chemical reaction involving the absorbent. However, it appears that many chemical pathways of destruction are involved because although complete destruction of the volatile anesthetic might continue, the production of carbon monoxide nevertheless would wane. The increasing emergence of anesthetic in the stream issuing from the absorbent correlated with decreasing emission of carbon monoxide.
Effect of Anesthetic Concentration on the Concentration of Carbon Monoxide Produced: Increasing the concentration of anesthetic directed through absorbent nearly proportionately increased the peak levels of carbon monoxide reached. The areas under the curves did not differ as much as the peak heights because the higher peak levels were not sustained.
Discussion
Although our artificial system will not necessarily reflect absolute concentrations of carbon monoxide that might be produced from these anesthetics being used in a conventional anesthesia machine/ gas delivery system, we believe it will reflect the relative production resulting from the changes in the factors examined (e.g., dryness, temperature, anesthetic type and concentration). We have chosen to examine clinically-relevant concentrations of presently available anesthetics and clinically relevant temperatures. The higher temperatures chosen are those that can be obtained in dosed circuit anesthesia.’ Although dry soda lime and Baralyme are not commonly used, these normally ‘wet’ absorbents (normal water content) may become dry if higher flow rates are applied or if the inflow of oxygen is continued overnight or over a weekend. (4) Thus, although the absolute levels of carbon monoxide developed in these systems will not be replicated in clinical practice, they indicate relative levels that might be anticipated.
We find that temperature, dryness of absorbent, type of absorbent, choice of anesthetic, and anesthetic concentration each can influence the concentration of carbon monoxide that can result from chemical degradation of the anesthetic by the absorbent. An increase in temperature and absorbent dryness increases anesthetic degradation and resultant production of carbon monoxide. However, of these two factors, dryness is, by far, more important than temperature. Baralyme produces more carbon monoxide than soda lime, particularly with a very (abnormally) low water content. More carbon monoxide is produced from desflurane and enflurane than from isoflurane and, for practical purposes, Do carbon monoxide is produced from halothane or sevoflurane.
Explains Case Reports
Our results can explain the anecdotal reports of appreciable levels of carbon monoxide appearance, in some cases enough to cause toxicity. The rarity of the reports is explained by our finding that production of appreciable levels of carbon monoxide requires nearly complete drying out of the absorbent. Such drying is rare, or at least unusual. The finding that higher levels usually appear in the first cases anesthetized on a Monday (5) may be explained by drying of absorbent over the weekend, which would be particularly Rely if oxygen flow through the absorbent has been unintentionally continued all weekend. 100% oxygen flow at the end of a case in order to assure adequate oxygenation before moving the patient to the post anesthesia care unit is common. If left on after the last case on Friday, this completely dry gas would be directed through the top end of the absorbent (6,7) (the freshest portion) and, with the prolonged period of a weekend, would dry the absorbent. The present study shows that more carbon monoxide is likely to be produced from such dry absorbent. Similarly, the finding that carbon monoxide levels roughly correlate with the length of time that an absorbent canister is in place could be explained by drying of the absorbent by the use of high inflow rates. Variation in inflow rates applied could explain the limited nature of the correlation found (i.e., could explain the variability of the results).
The report by Lentz found carbon monoxide in a patient given desflurane on Monday morning using a machine in which Baralyme was the absorbent.’ The hospital involved subsequently discontinued the use of Baralyme, substituting soda lime (unpublished report). Although desflurane continues to be used at this hospital, no subsequent cases of significant levels of carbon monoxide have arisen. The findings reported here would explain this occurrence. Baralyme causes a greater conversion of anesthetic to carbon monoxide than does soda lime, particularly if these absorbents contain abnormally low levels of water. Finally, most previous reports implicated enflurane, and a recent report has implicated desflurane, a finding consistent with our data indicating that absorbents acting on these agents produce higher levels of carbon monoxide than those resulting from degradation of the other volatile anesthetics.
Recommendations:
The findings reported here lead to specific recommendations for the avoidance of carbon monoxide production from the interaction of potent volatile anesthetics with carbon dioxide absorbent:
1. Ensure the use of standard absorbents containing the full complement of water. Use of relatively low fresh gas inflow rates for the majority of procedures should provide a sustained level of water content in the absorbent to avoid carbon monoxide production.
2. A corollary to (1) is to discontinue the use of high inflow rates when they are no longer needed (e.g. after equilibration of the patient to the desired maintenance level of volatile anesthetic). If an inflow of dry gas has been accidentally continued over a weekend, replace the absorbent with fresh absorbent. Data regarding the potential impact in this context of a continued flow of gas over one night are not yet available.
3. The use of soda lime rather than Baralyme should decrease the likelihood of production of appreciable amounts of carbon monoxide both because Baralyme that is completely dry produces more carbon monoxide, and because preservation of some of the water content (partial wetting) of soda lime reduces production of carbon monoxide more than the partial wetting of Baralyme.
These data and additional details are being submitted in a formal manuscript to a peer-reviewed anesthesia journal with the intention of the earliest possible publication.
Dr. Fang is a Research Fellow and Dr. Eger is Professor in the Department of Anesthesia, University of California, San Francisco, CA. Dr. Eger is also a consultant for Ohmeda.
This research was supported by grants from Ohmeda, Pharmaceutical Products Div., Inc., and from The Anesthesia Research Foundation.
References
1. Lentz R: CO poisoning during anesthesia poses puzzles. Anesthesia Patient Safety Foundation Newsletter 1994; 9:13-14.
2. Moon R: Cause of CO poisoning, relation to halogenated agents still not clear. Anesthesia Patient Safety Foundation Newsletter 1994; 9:13-16.
3. Moon R, Ingram C, Brunner E, Meyer A: Spontaneous generation of carbon monoxide within anesthetic circuits. Anesthesiology 1991; 75:A873 (Abstract).
4. Strum D, Eger El 11: The degradation, absorption, and solubility of volatile anesthetics in soda lime depend on water content. Anesthe Analg 1994; 78:30348.
5. Moon R, Meyer A, Scott D, Fox E, Millington D, Norwood D: Intraoperative carbon monoxide toxicity. Anesthesiology 1990; 73:A1049 (Abstract).
6. Harper M, Eger El U: A comparison of the efficiency of three anesthesia circle systems. Anesth Analg 1976; 55:724-729.
7. Eger El 11, Ethans C: The effects of inflow, overflow and valve placement on economy of the circle system. Anesthesiology 1968; 29:93-100.

